Ipca Laboratories Ltd. Corporate Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
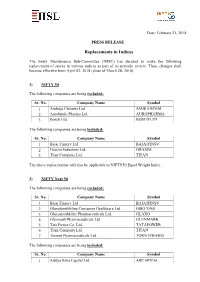
Replacements in Indices
Date: February 21, 2018 PRESS RELEASE Replacements in Indices The Index Maintenance Sub-Committee (IMSC) has decided to make the following replacement of stocks in various indices as part of its periodic review. These changes shall become effective from April 02, 2018 (close of March 28, 2018). 1) NIFTY 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Ambuja Cements Ltd. AMBUJACEM 2 Aurobindo Pharma Ltd. AUROPHARMA 3 Bosch Ltd. BOSCHLTD The following companies are being included: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 Grasim Industries Ltd. GRASIM 3 Titan Company Ltd. TITAN The above replacements will also be applicable to NIFTY50 Equal Weight Index. 2) NIFTY Next 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 GlaxoSmithkline Consumer Healthcare Ltd. GSKCONS 3 Glaxosmithkline Pharmaceuticals Ltd. GLAXO 4 Glenmark Pharmaceuticals Ltd. GLENMARK 5 Tata Power Co. Ltd. TATAPOWER 6 Titan Company Ltd. TITAN 7 Torrent Pharmaceuticals Ltd. TORNTPHARM The following companies are being included: Sr. No. Company Name Symbol 1 Aditya Birla Capital Ltd. ABCAPITAL Sr. No. Company Name Symbol 2 Ambuja Cements Ltd. AMBUJACEM 3 Aurobindo Pharma Ltd. AUROPHARMA 4 Bosch Ltd. BOSCHLTD 5 General Insurance Corporation of India GICRE 6 L&T Finance Holdings Ltd. L&TFH 7 SBI Life Insurance Company Ltd. SBILIFE 3) NIFTY 500 The following companies are being excluded: Sr. No. Company Name Symbol 1 Adani Enterprises Ltd. ADANIENT 2 Ahluwalia Contracts (India) Ltd. AHLUCONT 3 Apar Industries Ltd. APARINDS 4 AstraZenca Pharma India Ltd. ASTRAZEN 5 Corporation Bank CORPBANK 6 Dalmia Bharat Ltd. -

50 KW Offer Solar Copy 2
21st Century Enviro Engineers Pvt.Ltd. Plot No. 120(10 Marla), COMPANY Industrial Area Phase II Chandigarh - 160002, India PROFILE www.21stcenturyenviro.com INTRODUCTION 21st CENTURY ENVIRO ENGINEERS PVT. LTD. is a Company dealing in the field of Environmental Engineering related activities. We are Registered Environmental Consultants of Pollution Control Board to Supply ETP’s, STP’s, APCD’s, Incinerators, Water Treatment Plants, Reverse Osmosis, Evaporators, Solid Waste Management and Rain Water Harvesting and to carry out EIA Studies . The Directors of the company are young Technocrats having versatile experience in this field. We have a back up of highly qualified and experienced technical team having versatile experience in Designing, Erection, Commissioning and Operation of different types of Effluent Treatment Plants. Our Managing Director himself is a Chemical Engineer with almost 25 Years Experience in this line and Technical Director is PhD. in Environmental Science with almost 30 Years working experience on various types of effluent treatment Technologies. Our team comprises of almost 150 people from different backgrounds e.g. Chemical, Environmental, Mechanical, Instrumentation, Civil, Accounts, Purchase, Marketing etc. We have our Marketing/ Design Office in Chandigarh and regional offices in Dhaka, Mumbai, New Delhi, Sikkim etc. and have our own testing Laboratory for Effluent/Water Testing and to study treatability of effluent on pilot scale. We have our own full-fledged manufacturing unit of Pollution Control Equipments in Village Kunjhal Baddi (HP). We also undertake annual operation and maintenance contracts and liasining services for the systems supplied by us. We are also Registered with CREST (Chandigarh Renewable Energy & Technology Promotion Society) and SECI (Solar Energy Corporation of India) for supplying Online/Offline Solar systems in Chandigarh, Himachal Pradesh, Uttarakhand, J&K, Punjab, Haryana, Delhi, Bihar etc. -

Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock
Visit us at www.sharekhan.com November 04, 2015 Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock Update >> Ipca Laboratories Stock Update >> Skipper Viewpoint >> Lloyd Electric & Engineering For Private Circulation only REGISTRATION DETAILS Regd Add: Sharekhan Limited, 10th Floor, Beta Building, Lodha iThink Techno Campus, Off. JVLR, Opp. Kanjurmarg Railway Station, Kanjurmarg (East), Mumbai – 400042, Maharashtra. Tel: 022 - 61150000. Sharekhan Ltd.: SEBI Regn. Nos. BSE - INB/INF011073351 ; BSE- CD ; NSE - INB/ INF231073330 ; CD-INE231073330 ; MSEI - INB/INF261073333 ; CD-INE261073330 ; DP - NSDL-IN-DP-NSDL-233-2003 ; CDSL-IN-DP-CDSL-271-2004 ; PMS- INP000000662 ; Mutual Fund-ARN 20669 ; Commodity trading through Sharekhan Commodities Pvt. Ltd.: MCX-10080 ; (MCX/TCM/CORP/0425) ; NCDEX- 00132 ; (NCDEX/TCM/CORP/0142) ; NCDEX SPOT-NCDEXSPOT/116/CO/11/20626 ; For any complaints email at [email protected] ; Disclaimer: Client should read the Risk Disclosure Document issued by SEBI & relevant exchanges and Do’s & Don’ts by MCX & NCDEX and the T & C on www.sharekhan.com before investing. investor’s eye stock update Marico Reco: Buy Stock Update Enhanced focus to improve volume growth; maintain Buy CMP: Rs399 Company details Key points Price target: Rs460 Mixed operating performance: During Q2FY2016, Marico’s revenue grew by 4% to Rs1,485.4 crore, entirely driven by a 4% volume growth (domestic business’ volume Market cap: Rs25,735 cr growth stood at 5.5%). The gross profit margin (GPM) improved by almost 500BPS to 52 week high/low: Rs466/299 49.3% on the back of ~30% decline in the copra prices and 33% decline in the prices of liquid paraffin. -

Inner 25 India Pharma & Healthcare Fund
Modera erate tely Mod High to e H w at ig o er h L d o M V e r y w H Tata India Pharma & Healthcare Fund o i L g (An open ended equity scheme investing in Pharma and Healthcare Services Sector) h Riskometer Investors understand that their principal As on 30th June 2021 PORTFOLIO will be at Very High Risk INVESTMENT STYLE Company name No. of Market Value % of Company name No. of Market Value % of Primarily focuses on investment in at least 80% of its net Shares Rs. Lakhs Assets Shares Rs. Lakhs Assets assets in equity/equity related instruments of the companies in the Pharma & Healthcare sectors in India. Equity & Equity Related Total 55184.24 97.88 Glenmark Pharmaceuticals Ltd. 167000 1089.76 1.93 INVESTMENT OBJECTIVE Healthcare Services Sanofi India Ltd. 13000 997.39 1.77 The investment objective of the scheme is to seek long Apollo Hospitals Enterprise Ltd. 70500 2551.99 4.53 Gland Pharma Ltd. 19662 673.29 1.19 term capital appreciation by investing atleast 80% of its Fortis Healthcare Ltd. 795000 1935.03 3.43 Laurus Labs Ltd. 90000 619.79 1.10 net assets in equity/equity related instruments of the companies in the pharma & healthcare sectors in Syngene International Ltd. 265000 1545.75 2.74 India.However, there is no assurance or guarantee that the investment objective of the Scheme will be Narayana Hrudayalaya Ltd. 301420 1483.74 2.63 Other Equities^ 1186.14 2.10 achieved.The Scheme does not assure or guarantee any Metropolis Healthcare Ltd. -

Ipca Laboratories (IPCLAB)
Ipca Laboratories (IPCLAB) CMP: | 2040 Target: | 2290 (12%) Target Period: 12 months HOLD May 31, 2021 Upbeat guidance, execution remains key Q4 revenues remained subdued growing just 3.8% YoY to | 1115 crore. Strong YoY growth of 19.7% in export formulations to | 338 crore was partly Particulars offset by API sales decline of 5.5% YoY to | 260 crore. Domestic Particular Amount formulations remained flat at | 434 crore vs. | 431 crore in Q4FY20. EBITDA Market Capitalisation | 25873 crore margins improved 484 bps YoY to 20.5% due to better gross margins and Debt (FY21) | 267 crore lower other expenditure. EBITDA grew 35.8% YoY to | 229 crore. PAT grew Cash (FY21) | 365 crore 87.5% YoY to | 161 crore (I-direct estimate: | 204 crore). Delta vis-à-vis EV | 25775 crore EBITDA was due to higher other income, lower depreciation and tax rate. 52 week H/L (|) 2456/1467 Equity capital | 25.4 crore Update Result Export formulations main catalyst for growth Face value | 2 Price performance Growth in export formulations (29% of FY21 revenues) was on the back of growth in both international generics and international branded 2500 14000 formulations. The international anti-malarial institutional business has also 12000 2000 contributed substantially to overall exports growth. US traction will take 10000 longer than earlier estimated due to USFDA import alerts for the Ratlam 1500 8000 facility that is the only API source for Silvassa and Pithampur formulations 1000 6000 plants along with Silvassa and Pithampur (Indore) plants that are specifically 4000 500 earmarked for the US business, besides third party sales. -

Wardha District, Maharashtra
1765/DBR/2013 भारत सरकार जल संसाधन मंत्रालय कᴂ द्रीय भूजल बो셍ड GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD महाराष्ट्र रा煍य के अंत셍डत वधाड जजले की भूजल ववज्ञान जानकारी GROUND WATER INFORMATION WARDHA DISTRICT, MAHARASHTRA By 饍वारा Afaque Manzar आफ़ाक़ मंजर Scientist-B वैज्ञाननक- ख ´Ö¬µÖ •Öê¡Ö, •ÖÖ•Ö¯Öã¸ü CENTRAL REGION NAGPUR 2013 WARDHA DISTRICT AT A GLANCE 1. GENERAL INFORMATION Geographical Area : 6310 sq. km. Administrative Divisions : Taluka-8; Wardha, Deoli, Selu, Arvi, Ashti, Karanja, Hinganghat, Samudrapur. Villages : 1361 Population (2001 Census) : 12,30,640 Normal Annual Rainfall 985 mm to 1100 mm 2. GEOMORPHOLOGY Major Physiographic unit : Two; Northern Hills and Southern Plains Major Drainage : One; Wardha 3. LAND USE (2008-09) Forest Area : 590.13 sq. km. Net Area Sown : 187.00 sq. km. Cultivable Area : 2568.41 sq. km. 4. SOIL TYPE Black or Dark Brown soil viz., Kali, Morand, Khardi and Bardi. 5. PRINCIPAL CROPS (2008-09) Wheat 106.56 sq. km. Jowar 224.53 sq. km. Cotton 845.26 sq. km Total Pulses : 341.61 sq. km 6. IRRIGATION BY DIFFERENT SOURCES (2006-07 MI CENSUS) - Nos./Potential Created (ha) Dugwells : 47294/99920 Borewells : 70/176 Surface Flow Schemes : 607/1702 Surface Lift Schemes : 1184/3339 Net Potential Created : 105137 ha 7. GROUND WATER MONITORING WELLS (As on Nov 2012) Dugwells : 38 Piezometers : 17 8. GEOLOGY Recent : Alluvium Upper Cretaceous-Lower Eocene: Basalt (Deccan Trap) Middle Cretaceous : Infra-trappean beds 9. -

Bugs R All December 2012 FINAL
ISSN 2230 – 7052 No. 19, December 2012 Bugs R All Newsletter of the Invertebrate Conservation & Information Network of South Asia IUCN Species Survival Commission: Joint vision, goal and objecves of the SSC and IUCN Species Programme for 2013-16 The work of the SSC is guided by the Vision of: 2. Analysing the threats to biodiversity A just world that values and conserves nature through To analyse and communicate the threats to biodiversity posive acon to reduce the loss of diversity of life on and disseminate informaon on appropriate global earth. conservaon acons; 3. Facilitang and undertaking conservaon acon The overriding goal of the Commission is: To facilitate and undertake acon to deliver biodiversity- The species exncon crisis and massive loss of based soluons for halng biodiversity decline and catalyse biodiversity are universally adopted as a shared measures to manage biodiversity sustainably and prevent responsibility and addressed by all sectors of society species‟ exncons both in terms of policy change and taking posive conservaon acon and avoiding negave acon on the ground; impacts worldwide. 4. Convening experAse for biodiversity conservaon To provide a forum for gathering and integrang the Main strategic objecves: knowledge and experience of the world‟s leading experts For the intersessional period 2013–2016, the SSC, working on species science and management, and promong the in collaboraon with members, naonal and regional acve involvement of subsequent generaons of species commiees, other Commissions and the Secretariat, will conservaonists. pursue the following key objecves in helping to deliver IUCN‟s “One Programme” commitment: More informaon is available in the IUCN Species 1. -

Pharma Sector Report -Sept’20
Mutual Fund: Pharma Sector Report -Sept’20 Private & confidential. Only for limited circulation. Contact us: [email protected] Executive Summary: Since the outbreak of the Coronavirus crisis, the pharma sector stepped into the spotlight. Long overdue investments have been flowing towards the sector and even pharma funds in the mutual fund industry have begun to outperform. Nevertheless, there is still a clear need for government spending in this sector. Compared to the US, which has the highest per capita spending on healthcare at $5000, India spends merely ~ $50 per capita. The current situation presents the Indian government with a perfect opportunity to increase its expenditure in this sector, to facilitate its growth and fast-track its progress. Few points about the Pharma Funds: The market capitalization of the entire pharma & healthcare industry is ~ Rs. 10 lac crs, whereas the total size of pharma funds in the mutual fund is ~Rs. 10k crs, translating to ~ 1% of the entire sector in value terms. (This excludes the pharma holdings in other categories). There are 43 pharma & healthcare companies that form part of the Top 500 companies. Out of these, pharma funds have an exposure to 36 companies. There are 11 companies in the Top 100; 11 in the Midcap space (101-250) and 14 Smallcaps (251-500). The 7 companies that do not form part of the pharma funds’ portfolios are Caplin Point Lab, Glenmark Pharma, Granules India, Piramal Enterprises, Poly Medicure, Suven Pharma and Wockhardt Ltd. Cipla Ltd., Divis Laboratories Ltd. and Dr. Reddy’s Laboratories Ltd. are the only stocks that form part of every pharma fund. -

How Coal Mining Is Trashing Tigerland
Author Contact Ashish Fernandes Ashish Fernandes [email protected] Research coordination & North Karanpura case study Nandikesh Sivalingam Kanchi Kohli [email protected] Research Photo Editor Aishwarya Madineni, Vikal Samdariya, Arundhati Sudhanshu Malhotra Muthu and Preethi Herman Design GIS Analysis Aditi Bahri Ecoinformatics Lab, ATREE (Kiran M.C., Madhura Cover image Niphadkar, Aneesh A., Pranita Sambhus) © Harshad Barve / Greenpeace Acknowledgments Image Sudiep Shrivastava for detailed inputs on the Forests of Sanjay Dubri Tiger Hasdeo-Arand and Mandraigarh sections, Kishor Reserve near Singrauli coalfield Rithe for inputs on the Wardha and Kamptee © Dhritiman Mukherjee / Greenpeace sections, Bulu Imam and Justin Imam for their expertise on the North Karanpura section, Biswajit Printed on 100% recycled paper. Mohanty for feedback on the Talcher and Ib Valley sections and Belinda Wright for feedback on the Sohagpur and Singrauli sections. CONTENTS Executive Summary 01 9. Hasdeo-Arand (Chhattisgarh) 51 10. West Bokaro (Jharkhand) 55 Introduction 09 Central India,Tigers, Corridors and Coal 11. North Karanpura (Jharkhand) 60 How Coal is Trashing Tigerland 17 Case Study I 63 The North Karanpura Valley - On the edge Methodology 21 12. Wardha (Maharashtra) 00 Coalfield Analysis 25 13. Kamptee (Maharashtra) 00 1. Singrauli (Madhya Pradesh - Chhattisgarh) 27 Case Study II 87 2. Sohagpur (Madhya Pradesh - Chhattisgarh) 33 Chandrapur’s tigers - Encircled by coal 3. Sonhat (Chhattisgarh) 35 4. Tatapani (Chhattisgarh) 37 Alternatives: Efficiency and Renewables 101 5. Auranga (Jharkhand) 39 References 109 6. Talcher (Odisha) 41 Glossary 7. Ib Valley (Odisha) 47 110 8. Mandraigarh (Chhattisgarh) 49 Endnotes 111 EXECUTIVE SUMMARY As India’s national animal, the Royal Bengal Tiger Panthera tigris has ostensibly been a conservation priority for current and past governments. -

List of 3Rd Party Manufacturer of Pre-Qualified/Registered Firms with PPT Hospital
List of 3rd party Manufacturer of Pre-qualified/Registered Firms with PPT Hospital SL. Name of the Parent Firm Name of the 3rd party manufacturer NO A B C 1 M/s AKUMENTIS HEALTHCARE LTD. (i) M/S Unimark Healthcare Ltd (ii) M/S Applied Communication & Controls (iii) Maxcure Nutravedics Ltd (iv) Akums Drugs & Pharmaceuticals Ltd 2 M/s ALCON LABORATORIES(INDIA)PVT.LTD (i) M/S Wintac Limited 3 M/s ABBOTT HEALTHCARE PVT. LTD. (i) Akums Drugs & Pharmaceuticals Ltd (ii) Medibios Laboratories Pvt. Ltd. (iii) Tristar Formulations Pvt. Ltd. (iv) Hetero Labs Ltd (v) M/S Aqua Vitoe Laboratories 4 M/s ABBOTT INDIA LIMITED. (i) Acme Formulation Pvt Ltd (ii) Revenbhel Healthcare Pvt. Ltd. 5 M/s CENTAUR PHARMACEUTICALS PVT. LTD. (i) M/S Pure & Cure Healthcare Pvt. Ltd. (ii) M/S The Madras Pharmaceuticals (iii) M/S Akums Drugs & Pharmaceuticals Limited 6 M/S CIPLA LIMITED (i) Akums Drugs & Pharmaceuticals Ltd (ii) BDR Pharmaceuticals International Pvt. Ltd. (iii) M/S Medispray Laboratories Pvt. Ltd. (iv) M/S Golden Cross Pharma Pvt. Ltd. (v) M/S Tirupati Medicare Limited (vi) M/S Mepromax Lifesciences PVT Ltd. (vii) M/S Virchow Biotech Private Limited (viii) Hetero Labs Ltd (ix) Pegasus Farmaco Iindia Pvt. Ltd. (x) Meditab Specialities Pvt. Ltd. 7 M/s DR. REDDYS LABORATORIES LTD (i) Bdr Pharmaceuticals Ltd (ii) Naprod Lifesciences Pvt. Ltd (iii) Hetero Labs Ltd (iv) Natco Pharma Ltd (v) Amgen Manufacturing Limited 8 M/s FOURRTS (INDIA) LABORATORIES PVT. (i) M/S Swiss Garnier Biotech (ii) M/S Sun Glow Pharmaceuticals Private Limited 9 M/s GLOBUS REMEDIES (i) Park Pharmaceuticals (ii) Baxil Pharma Pvt. -

People for Animals, Wardha
People For Animals, Wardha Annual Report for the year 2017-18 PEOPLE FOR ANIMALS, WARDHA Page | 1 CONTENTS Sr. Section Page No Number 1. Report of the Officer-in-charge 1. 2. History of the Rescue Centre 1. 3. Vision 1. 4. Mission 1. 5. Objective 1. 6. About us 2. 7. Organizational Chart 7. 8. Human Resources for management of Rescue Centre 7. 9. Capacity Building of Rescue Centre Personnel 8. 10. Rescue Centre Advisory Committee 8. 11. Health Advisory Committee 9. 12. Statement of income and expenditure of the Rescue Centre 9. 13. Daily feed Schedule of animals 9. 14. Vaccination Schedule of animals 10. 15. De-worming Schedule of animals 11. Page | 2 Sr. Section Page No Number 16. Disinfection Schedule 11. 17. Health Check-up of employees for zoonotic diseases 13. 18. Development Works carried out in the Rescue Centre during the 13. year 19. Important Events and happenings 14. 20. Seasonal special arrangements for upkeep of animals 15. 21. Research Work carried out and publications 15. 22. Rescue and Rehabilitation of the wild Animals 15. 23. Annual Inventory of animals 18. 24. Mortality of animals. 20. 25. Status of the Compliance with conditions stipulated by the 22. Central Zoo Authority Page | 3 1. Report of the Officer-in-charge People For Animals, wardha animal centre is a non granted organization established in 1999 with the aim of provide safe shelter & medical treatment for the animals in need. In this financial year we rescued numbers of wild animals, some of them released in their natural habitat. -

Weights for New Portfolio of India Small Cap Index (ISCIN) - As on March 12, 2013 S No Ticker Name ISIN SEDOL Weights 1 FB in Equity Federal Bank Ltd
Weights for New Portfolio of India Small Cap Index (ISCIN) - As on March 12, 2013 S No Ticker Name ISIN SEDOL Weights 1 FB IN Equity Federal Bank Ltd. INE171A01011 6139845 4.00% 2 APHS IN Equity Apollo Hospitals Enterprise Ltd. INE437A01024 6273583 3.52% 3 ABNL IN Equity Aditya Birla Nuvo Ltd. INE069A01017 6100421 3.43% 4 TTCH IN Equity Tata Chemicals Ltd. INE092A01019 6101167 3.26% 5 MMFS IN Equity Mahindra & Mahindra Financial Services Ltd. INE774D01024 B8F8822 3.25% 6 TGBL IN Equity Tata Global Beverages Ltd. INE192A01025 6121488 3.09% 7 HPCL IN Equity Hindustan Petroleum Corp. Ltd. INE094A01015 6100476 2.95% 8 EXID IN Equity Exide Industries Ltd. INE302A01020 B1D3ZC9 2.86% 9 RCAPT IN Equity Reliance Capital Ltd. INE013A01015 6101082 2.44% 10 UNTP IN Equity United Phosphorus Ltd. INE628A01036 B0L0W35 2.32% 11 PLNG IN Equity Petronet LNG Ltd. INE347G01014 B00KT68 2.24% 12 AL IN Equity Ashok Leyland Ltd. INE208A01029 B01NFT1 2.20% 13 UT IN Equity Unitech Ltd. INE694A01020 B17MRV5 2.14% 14 CRG IN Equity Crompton Greaves Ltd. INE067A01029 B1B90H9 2.08% 15 OBC IN Equity Oriental Bank of Commerce INE141A01014 6121507 2.01% 16 IPCA IN Equity IPCA Laboratories Ltd. INE571A01020 6433473 1.96% 17 JUBI IN Equity Jubilant FoodWorks Ltd. INE797F01012 B3PRM66 1.94% 18 MRF IN Equity MRF Ltd. INE883A01011 6214128 1.88% 19 ALBK IN Equity Allahabad Bank INE428A01015 6708289 1.85% 20 STR IN Equity Strides Arcolab Ltd. INE939A01011 6690535 1.84% 21 DITV IN Equity Dish TV India Ltd. INE836F01026 B1RMW32 1.78% 22 JKBK IN Equity Jammu & Kashmir Bank Ltd.