View Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
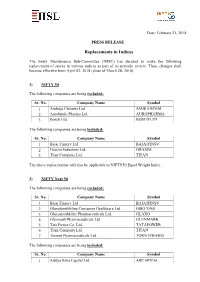
Replacements in Indices
Date: February 21, 2018 PRESS RELEASE Replacements in Indices The Index Maintenance Sub-Committee (IMSC) has decided to make the following replacement of stocks in various indices as part of its periodic review. These changes shall become effective from April 02, 2018 (close of March 28, 2018). 1) NIFTY 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Ambuja Cements Ltd. AMBUJACEM 2 Aurobindo Pharma Ltd. AUROPHARMA 3 Bosch Ltd. BOSCHLTD The following companies are being included: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 Grasim Industries Ltd. GRASIM 3 Titan Company Ltd. TITAN The above replacements will also be applicable to NIFTY50 Equal Weight Index. 2) NIFTY Next 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 GlaxoSmithkline Consumer Healthcare Ltd. GSKCONS 3 Glaxosmithkline Pharmaceuticals Ltd. GLAXO 4 Glenmark Pharmaceuticals Ltd. GLENMARK 5 Tata Power Co. Ltd. TATAPOWER 6 Titan Company Ltd. TITAN 7 Torrent Pharmaceuticals Ltd. TORNTPHARM The following companies are being included: Sr. No. Company Name Symbol 1 Aditya Birla Capital Ltd. ABCAPITAL Sr. No. Company Name Symbol 2 Ambuja Cements Ltd. AMBUJACEM 3 Aurobindo Pharma Ltd. AUROPHARMA 4 Bosch Ltd. BOSCHLTD 5 General Insurance Corporation of India GICRE 6 L&T Finance Holdings Ltd. L&TFH 7 SBI Life Insurance Company Ltd. SBILIFE 3) NIFTY 500 The following companies are being excluded: Sr. No. Company Name Symbol 1 Adani Enterprises Ltd. ADANIENT 2 Ahluwalia Contracts (India) Ltd. AHLUCONT 3 Apar Industries Ltd. APARINDS 4 AstraZenca Pharma India Ltd. ASTRAZEN 5 Corporation Bank CORPBANK 6 Dalmia Bharat Ltd. -

50 KW Offer Solar Copy 2
21st Century Enviro Engineers Pvt.Ltd. Plot No. 120(10 Marla), COMPANY Industrial Area Phase II Chandigarh - 160002, India PROFILE www.21stcenturyenviro.com INTRODUCTION 21st CENTURY ENVIRO ENGINEERS PVT. LTD. is a Company dealing in the field of Environmental Engineering related activities. We are Registered Environmental Consultants of Pollution Control Board to Supply ETP’s, STP’s, APCD’s, Incinerators, Water Treatment Plants, Reverse Osmosis, Evaporators, Solid Waste Management and Rain Water Harvesting and to carry out EIA Studies . The Directors of the company are young Technocrats having versatile experience in this field. We have a back up of highly qualified and experienced technical team having versatile experience in Designing, Erection, Commissioning and Operation of different types of Effluent Treatment Plants. Our Managing Director himself is a Chemical Engineer with almost 25 Years Experience in this line and Technical Director is PhD. in Environmental Science with almost 30 Years working experience on various types of effluent treatment Technologies. Our team comprises of almost 150 people from different backgrounds e.g. Chemical, Environmental, Mechanical, Instrumentation, Civil, Accounts, Purchase, Marketing etc. We have our Marketing/ Design Office in Chandigarh and regional offices in Dhaka, Mumbai, New Delhi, Sikkim etc. and have our own testing Laboratory for Effluent/Water Testing and to study treatability of effluent on pilot scale. We have our own full-fledged manufacturing unit of Pollution Control Equipments in Village Kunjhal Baddi (HP). We also undertake annual operation and maintenance contracts and liasining services for the systems supplied by us. We are also Registered with CREST (Chandigarh Renewable Energy & Technology Promotion Society) and SECI (Solar Energy Corporation of India) for supplying Online/Offline Solar systems in Chandigarh, Himachal Pradesh, Uttarakhand, J&K, Punjab, Haryana, Delhi, Bihar etc. -

United Spirits Limited Corporate Identity Number: L01551KA1999PLC024991 Registered Office: ‘UB Tower’, #24, Vittal Mallya Road, Bangalore - 560 001
United Spirits Limited Corporate Identity Number: L01551KA1999PLC024991 Registered Office: ‘UB Tower’, #24, Vittal Mallya Road, Bangalore - 560 001. Tel: +91 80 3985 6500; Fax: +91 80 3985 6862; www.unitedspirits.in, Email: [email protected] NOTICE NOTICE IS HEREBY GIVEN OF THE FIFTEENTH ANNUAL GENERAL MEETING (“AGM”) of United Spirits Limited (the “Company”) to be held at Level 1, UB Tower, #24, Vittal Mallya Road, Bangalore 560 001 on Tuesday, September 30, 2014 at 2.30 p.m. for the following purposes: ORDINARY BUSINESS 1. To receive, consider and adopt the Audited Statement of Profit and Loss for the financial year ended March 31, 2014, the Balance Sheet as at that date and the Reports of the Directors and Auditors thereon. 2. To appoint a Director in place of Dr. Vijay Mallya (DIN: 00122890), who retires by rotation and being eligible, offers himself for re-appointment. 3. To consider and if thought fit, to pass with or without modification(s), the following resolution as an Ordinary Resolution: RESOLVED that the vacancy in the Board of Directors of the Company arising out of the retirement of Mr. Gilbert Ghostine (DIN: 06555302) who retires by rotation at this AGM and has not offered himself for re-appointment, not be filled up as of the current date. 4. To appoint Statutory Auditors and to fix their remuneration. To consider and if thought fit, to pass with or without modification(s), the following resolution as an Ordinary Resolution: RESOLVED that pursuant to the provisions of Section 139 of the Companies Act, 2013 and the rules made thereunder, and pursuant to the recommendation of the Audit Committee of the Board of Directors, M/s. -

Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock
Visit us at www.sharekhan.com November 04, 2015 Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock Update >> Ipca Laboratories Stock Update >> Skipper Viewpoint >> Lloyd Electric & Engineering For Private Circulation only REGISTRATION DETAILS Regd Add: Sharekhan Limited, 10th Floor, Beta Building, Lodha iThink Techno Campus, Off. JVLR, Opp. Kanjurmarg Railway Station, Kanjurmarg (East), Mumbai – 400042, Maharashtra. Tel: 022 - 61150000. Sharekhan Ltd.: SEBI Regn. Nos. BSE - INB/INF011073351 ; BSE- CD ; NSE - INB/ INF231073330 ; CD-INE231073330 ; MSEI - INB/INF261073333 ; CD-INE261073330 ; DP - NSDL-IN-DP-NSDL-233-2003 ; CDSL-IN-DP-CDSL-271-2004 ; PMS- INP000000662 ; Mutual Fund-ARN 20669 ; Commodity trading through Sharekhan Commodities Pvt. Ltd.: MCX-10080 ; (MCX/TCM/CORP/0425) ; NCDEX- 00132 ; (NCDEX/TCM/CORP/0142) ; NCDEX SPOT-NCDEXSPOT/116/CO/11/20626 ; For any complaints email at [email protected] ; Disclaimer: Client should read the Risk Disclosure Document issued by SEBI & relevant exchanges and Do’s & Don’ts by MCX & NCDEX and the T & C on www.sharekhan.com before investing. investor’s eye stock update Marico Reco: Buy Stock Update Enhanced focus to improve volume growth; maintain Buy CMP: Rs399 Company details Key points Price target: Rs460 Mixed operating performance: During Q2FY2016, Marico’s revenue grew by 4% to Rs1,485.4 crore, entirely driven by a 4% volume growth (domestic business’ volume Market cap: Rs25,735 cr growth stood at 5.5%). The gross profit margin (GPM) improved by almost 500BPS to 52 week high/low: Rs466/299 49.3% on the back of ~30% decline in the copra prices and 33% decline in the prices of liquid paraffin. -

Skillv/Smanagement
Vol:1 | Issue 3 | October 2014 For Private Circulation Only PAGES 60 ‘Andhra Pradesh gives importance to PPP model for tourism development’ Chandana Khan IAS Special Chief Secretary, Tourism & Culture, Archaeology & Museums, Archives & Youth Services & Sports, NCC, Govt. of AP Page no 45 Page no 24 HYDERABAD Trying to Regain the Lost Ground The Land of Royals and Heritage Page no 20 ‘We are trying to create a global tourism destination through the Spice Route’ P I Sheik Pareeth (IAS) Director, Kerala Tourism Page no 50 Owner-Manager relationship: Page no 10 Are Indian owners becoming assertive? New Executive Committee Members (2014-15) of SIHRA elected during the 63rd AGM, held in Chennai When students decide to step into the hospitality industry, the first thing they do is enroll into an IHM and attain the hospitality management Page no 30 degree. Each year, hundreds of students graduate out of IHMs where students are equipped to be Kerala Tourism: Unveiling managers. Does the hospitality industry really need so many managers or do they need skill- Hidden Treasures based professionals? Page no 38 Skill v/sManagement Need to revisit our priorities Page no 48 SIHRA NEWS | OCTOBER 2014 3 EDITOR & COO* Sheldon Santwan Head-Editorial Operations & Special Projects Content Sumit Jha Assistant Editor P Krishna Kumar 07 | Editor's Desk EDITORIAL BOARD - SIHRA Honorary Secretary 08 | News Round-up T Nataraajan Advisor 16 | FH RAI Annual Convention 2014: R Rangachari Secretary General An effort to make the Industry Voice Elina Maller Heard EDITORIAL -

Inner 25 India Pharma & Healthcare Fund
Modera erate tely Mod High to e H w at ig o er h L d o M V e r y w H Tata India Pharma & Healthcare Fund o i L g (An open ended equity scheme investing in Pharma and Healthcare Services Sector) h Riskometer Investors understand that their principal As on 30th June 2021 PORTFOLIO will be at Very High Risk INVESTMENT STYLE Company name No. of Market Value % of Company name No. of Market Value % of Primarily focuses on investment in at least 80% of its net Shares Rs. Lakhs Assets Shares Rs. Lakhs Assets assets in equity/equity related instruments of the companies in the Pharma & Healthcare sectors in India. Equity & Equity Related Total 55184.24 97.88 Glenmark Pharmaceuticals Ltd. 167000 1089.76 1.93 INVESTMENT OBJECTIVE Healthcare Services Sanofi India Ltd. 13000 997.39 1.77 The investment objective of the scheme is to seek long Apollo Hospitals Enterprise Ltd. 70500 2551.99 4.53 Gland Pharma Ltd. 19662 673.29 1.19 term capital appreciation by investing atleast 80% of its Fortis Healthcare Ltd. 795000 1935.03 3.43 Laurus Labs Ltd. 90000 619.79 1.10 net assets in equity/equity related instruments of the companies in the pharma & healthcare sectors in Syngene International Ltd. 265000 1545.75 2.74 India.However, there is no assurance or guarantee that the investment objective of the Scheme will be Narayana Hrudayalaya Ltd. 301420 1483.74 2.63 Other Equities^ 1186.14 2.10 achieved.The Scheme does not assure or guarantee any Metropolis Healthcare Ltd. -

Ipca Laboratories (IPCLAB)
Ipca Laboratories (IPCLAB) CMP: | 2040 Target: | 2290 (12%) Target Period: 12 months HOLD May 31, 2021 Upbeat guidance, execution remains key Q4 revenues remained subdued growing just 3.8% YoY to | 1115 crore. Strong YoY growth of 19.7% in export formulations to | 338 crore was partly Particulars offset by API sales decline of 5.5% YoY to | 260 crore. Domestic Particular Amount formulations remained flat at | 434 crore vs. | 431 crore in Q4FY20. EBITDA Market Capitalisation | 25873 crore margins improved 484 bps YoY to 20.5% due to better gross margins and Debt (FY21) | 267 crore lower other expenditure. EBITDA grew 35.8% YoY to | 229 crore. PAT grew Cash (FY21) | 365 crore 87.5% YoY to | 161 crore (I-direct estimate: | 204 crore). Delta vis-à-vis EV | 25775 crore EBITDA was due to higher other income, lower depreciation and tax rate. 52 week H/L (|) 2456/1467 Equity capital | 25.4 crore Update Result Export formulations main catalyst for growth Face value | 2 Price performance Growth in export formulations (29% of FY21 revenues) was on the back of growth in both international generics and international branded 2500 14000 formulations. The international anti-malarial institutional business has also 12000 2000 contributed substantially to overall exports growth. US traction will take 10000 longer than earlier estimated due to USFDA import alerts for the Ratlam 1500 8000 facility that is the only API source for Silvassa and Pithampur formulations 1000 6000 plants along with Silvassa and Pithampur (Indore) plants that are specifically 4000 500 earmarked for the US business, besides third party sales. -
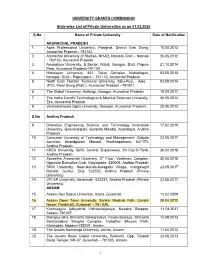
UNIVERSITY GRANTS COMMISSION State-Wise List of Private
UNIVERSITY GRANTS COMMISSION State-wise List of Private Universities as on 01.02.2020 S.No Name of Private University Date of Notification ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, 10.05.2013 Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai 26.05.2012 - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum 21.10.2014 Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, 03.05.2013 Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo 03.09.2014 (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 18.09.2017 7. The Indira Gandhi Technological & Medical Sciences University, 26.05.2012 Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. 20.06.2012 S.No Andhra Pradesh 9. Bharatiya Engineering Science and Technology Innovation 17.02.2019 University, Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh 10. Centurian University of Technology and Management, Gidijala 23.05.2017 Junction, Anandpuram Mandal, Visakhapatnam- 531173, Andhra Pradesh. 11. KREA University, 5655, Central, Expressway, Sri City-517646, 30.04.2018 Andhra Pradesh 12. Saveetha Amaravati University, 3rd Floor, Vaishnavi Complex, 30.04.2018 Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh 13. SRM University, Neerukonda-Kuragallu Village, mangalagiri 23.05.2017 Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) 14. VIT-AP University, Amaravati- 522237, Andhra Pradesh (Private 23.05.2017 University) ASSAM 15. Assam Don Bosco University, Azara, Guwahati 12.02.2009 16. Assam Down Town University, Sankar Madhab Path, Gandhi 29.04.2010 Nagar, Panikhaiti, Guwahati – 781 036. -

Activity Report 2009 – 2010
Activity Report 2009 – 2010 L V Prasad Eye Institute Kallam Anji Reddy Campus L V Prasad Marg, Banjara Hills Hyderabad 500 034, India Tel: 91 40 3061 2345 Fax: 91 40 2354 8271 e-mail: [email protected] L V Prasad Eye Institute Patia, Bhubaneswar 751 024 Orissa, India Tel: 91 0674 3989 2020 Fax: 91 0674 3987 130 e-mail: [email protected] L V Prasad Eye Institute G M R Varalakshmi Campus Door No: 11-113/1 Hanumanthawaka Junction Visakhapatnam 530 040 Andhra Pradesh, India Tel: 91 0891 3989 2020 Fax: 91 0891 398 4444 L V Prasad Eye Institute e-mail: [email protected] Excellence • Equity • Effi ciency Art with vision, for vision Artist-in-residence Sisir Sahana in his workshop on A view of the Art Gallery on Level 6 at Hyderabad LVPEI’s Kallam Anji Reddy campus, Hyderabad creating campus, where several works by Mr Surya Prakash, one of his signature glass sculptures. Inset: A piece from our senior artist-in-residence are on display. his latest collection, entitled “The long climb”. Inset: The hand that wields the paintbrush! L V Prasad Eye Institute Committed to excellence and equity in eye care Activity Report April 2009 – March 2010 Collaborating Centre for Prevention of Blindness L V Prasad Eye Institute, a not-for-profi t charitable organization, is governed by two trusts: Hyderabad Eye Institute and Hyderabad Eye Research Foundation. Donations to Hyderabad Eye Research Foundation are 175% exempt under section 35 (i) (ii) and donations made to Hyderabad Eye Institute are 50% exempt under section 80G of the Income Tax Act. -

Pharma Sector Report -Sept’20
Mutual Fund: Pharma Sector Report -Sept’20 Private & confidential. Only for limited circulation. Contact us: [email protected] Executive Summary: Since the outbreak of the Coronavirus crisis, the pharma sector stepped into the spotlight. Long overdue investments have been flowing towards the sector and even pharma funds in the mutual fund industry have begun to outperform. Nevertheless, there is still a clear need for government spending in this sector. Compared to the US, which has the highest per capita spending on healthcare at $5000, India spends merely ~ $50 per capita. The current situation presents the Indian government with a perfect opportunity to increase its expenditure in this sector, to facilitate its growth and fast-track its progress. Few points about the Pharma Funds: The market capitalization of the entire pharma & healthcare industry is ~ Rs. 10 lac crs, whereas the total size of pharma funds in the mutual fund is ~Rs. 10k crs, translating to ~ 1% of the entire sector in value terms. (This excludes the pharma holdings in other categories). There are 43 pharma & healthcare companies that form part of the Top 500 companies. Out of these, pharma funds have an exposure to 36 companies. There are 11 companies in the Top 100; 11 in the Midcap space (101-250) and 14 Smallcaps (251-500). The 7 companies that do not form part of the pharma funds’ portfolios are Caplin Point Lab, Glenmark Pharma, Granules India, Piramal Enterprises, Poly Medicure, Suven Pharma and Wockhardt Ltd. Cipla Ltd., Divis Laboratories Ltd. and Dr. Reddy’s Laboratories Ltd. are the only stocks that form part of every pharma fund. -

List of 3Rd Party Manufacturer of Pre-Qualified/Registered Firms with PPT Hospital
List of 3rd party Manufacturer of Pre-qualified/Registered Firms with PPT Hospital SL. Name of the Parent Firm Name of the 3rd party manufacturer NO A B C 1 M/s AKUMENTIS HEALTHCARE LTD. (i) M/S Unimark Healthcare Ltd (ii) M/S Applied Communication & Controls (iii) Maxcure Nutravedics Ltd (iv) Akums Drugs & Pharmaceuticals Ltd 2 M/s ALCON LABORATORIES(INDIA)PVT.LTD (i) M/S Wintac Limited 3 M/s ABBOTT HEALTHCARE PVT. LTD. (i) Akums Drugs & Pharmaceuticals Ltd (ii) Medibios Laboratories Pvt. Ltd. (iii) Tristar Formulations Pvt. Ltd. (iv) Hetero Labs Ltd (v) M/S Aqua Vitoe Laboratories 4 M/s ABBOTT INDIA LIMITED. (i) Acme Formulation Pvt Ltd (ii) Revenbhel Healthcare Pvt. Ltd. 5 M/s CENTAUR PHARMACEUTICALS PVT. LTD. (i) M/S Pure & Cure Healthcare Pvt. Ltd. (ii) M/S The Madras Pharmaceuticals (iii) M/S Akums Drugs & Pharmaceuticals Limited 6 M/S CIPLA LIMITED (i) Akums Drugs & Pharmaceuticals Ltd (ii) BDR Pharmaceuticals International Pvt. Ltd. (iii) M/S Medispray Laboratories Pvt. Ltd. (iv) M/S Golden Cross Pharma Pvt. Ltd. (v) M/S Tirupati Medicare Limited (vi) M/S Mepromax Lifesciences PVT Ltd. (vii) M/S Virchow Biotech Private Limited (viii) Hetero Labs Ltd (ix) Pegasus Farmaco Iindia Pvt. Ltd. (x) Meditab Specialities Pvt. Ltd. 7 M/s DR. REDDYS LABORATORIES LTD (i) Bdr Pharmaceuticals Ltd (ii) Naprod Lifesciences Pvt. Ltd (iii) Hetero Labs Ltd (iv) Natco Pharma Ltd (v) Amgen Manufacturing Limited 8 M/s FOURRTS (INDIA) LABORATORIES PVT. (i) M/S Swiss Garnier Biotech (ii) M/S Sun Glow Pharmaceuticals Private Limited 9 M/s GLOBUS REMEDIES (i) Park Pharmaceuticals (ii) Baxil Pharma Pvt. -

List of Registered Colonies Approved Under Hp Apartment
LIST OF REGISTERED COLONIES APPROVED UNDER H.P. APARTMENT AND PROPERTY REGULATION ACT, 2005 Sr. Name and Address of the Location of Project Licence No. & dated No Promoter . 1 2 3 4 Year-2005 (02) 1 Sh. Amarnath Aggarwal Village Dakru Pargana-Nalagarh, HIMUDA/LIC-1/2005 M/s Amar Nath Aggarwal Tehsil Nalagarh, District Solan, dated 5.10.2005. Builders Pvt. Ltd. Office 407, H.P. Motor Market, Mani Majra Chandigarh 2. Sh. Harish Aggarwal, M/s Mauza Katha Pargana, HIMUDA/LIC-2/2005 Mount View Group Housing Co. Dharampur( Baddi), Teshil dated 5.12.2005 Plot No.176, HPSIDC, Industrial Nalagarh, District Solan, H.P. Area, Baddi, District Solan, H.P. Year-2006 (11) 3. Sh. Lalit Jindal, M/s Hill View Village Bhatouli Kalan, Pargana- HIMUDA/LIC-3/2006 Infrastructure Pvt. Ltd-2048, Dharampur, Tehsil Nalagarh, dated 5.5.2006. Sector-15-C, Chandigarh, U.T. District Solan, H.P. 4. Sh. Amarnath Aggarwal, M/s Village Dakru Pargana-Nalagarh, HIMUDA/LIC-4/2006 Amar Nath Aggarwal Builders Tehsil Nalagarh, District Solan, dated 12.5.2006. Pvt. Ltd. Regd. Office, 407 H.P. Motor Market Manimajra Chandigarh and Central Office SCO-10-11, Sector, Panchkulla, Haryana 5. Sh. Ashwin Johar, M/s MDC Village Bhatouli Kalan, Pargana, HIMUDA/LIC-5/2006 Estates Pvt. Ltd. 319, Sector-21- Dharampur, Tehsil Nalagarh, dated 17.6.2006 A, Chandigarh U.T. District Solan, H.P. 6. Sh. Daleep Moudgil, M/s Omax Village Billanwali Gujran HIMUDA/LIC-6/2006 Construction Ltd.,7-Local Pargana-Dharampur, Tehsil dated 4.7.2006 Shopping Centre, Omax House, Nalagarh, District Solan, H.P.