Degradation of Microcystins Using Immobilized Microorganism Isolated in an Eutrophic Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Muse No. 14: Japanese Network of Museums for Peace
Muse no. 14: Japanese Network of Museums for Peace Newsletter: Feb, 2006 The Editorial Office: Kyoto Museum for World Peace, Ritsumeikan University 56-1 Kita-machi, Toji-in, Kita-ku, Kyoto City 603-8577 Japan Director: Ikuro Anzai. Curator: Masahiko Yamabe Editor: Kazuyo Yamane Illustrator: Erico Tosaki Tel: +81-075-465-8151. Fax: +81-075-465-7899. http://www.ritsumei.ac.jp The following is news on peace museums in Japan. Mr. Masahiko Yamabe, the curator of Kyoto Museum for World Peace, wrote news on big peace museums while Kazuyo Yamane of Grassroots House wrote news on small peace museums and other news. We hope you will enjoy reading them. The Fifth Conference of the Japanese Keiichiro Kaji, the member of the Network of Museums for Peace Center of the Tokyo Air Raid War Damages We held “the Fifth Nationwide Meeting of the National Network of Museums for 4. €34Exhibition organized by the Peace” at the conference room of the Kyoto Matsushiro Imperial Headquarters Museum for World Peace, Ritsumeikan Peace Memorial Museum” by Osamu University on December 3 (Sat) 13:00~18:00 Baba, the member of the nonprofit and December 4 (Sun) 9:00~12:00, 2005. organization for the Matsushiro The report on this event is as follows: Imperial Headquarters Peace Memorial Museum. 1. “Activities of the Auschwitz Peace Museum” by Masayuki Yamada, Auschwitz Peace Museum 2. “Women’s Active Museum(WAM) on War and Peace which was built 60 years after the end of the war ” by Eriko Ikeda, the member of the Women’s Museum on War and Peace 3. -

Aikawa Town Guide Book
Free Taking a look around Aikawa, So many things to do So many things to see! AikawaAikawa TownTown GuideGuide BookBook “Ai”“Ai” means means “Love”“Love”愛愛AiAi Aikawa is a town of love. So huge, so lovely! I’ m amazed Look, there are beautiful flowers at the power of the cascade! blooming throughout the year. (^o^) The beauty of this wonderful old residence shows the love of the carpenters of old. “450 years ago, a fierce battle took place and this stone marks the battleground.” I see... Town Areas and Sightseeing Spots Ken-O Expressway Wide Area Map Sagamihara IC Ken-O Expressway Sagamihara City Map of Places to See in AikawaKen-o-Do (Metropolitan Inter-City Expressway) Sagamihara City Aikawa Town Sagamihara Aikawa IC Horinouchi Uedana Kiyokawa Village Miyagase Dam: page 4 Hattori Dairy Farm: page 5 Mimase Park Atsugi City Battle of Mimase Pass: page 10 Mimase Park Athletic Ground Hattori Dairy Farm Rainbow Plaza Textile Association Prefectural Aikawa Park Shingen's Banner Tree Hinata Bridge Banda Station Hanbara Hinata Asari Grave Suzuki Confectionery and Shrine Miyagase Dam Aikawa Ohashi Bridge Boarding point for dam site pleasure boat Sagami Line Arts and Crafts Village Aikawa Forestry Association Mimase Prefectural Central Takata Bridge Water and Matsubazawa Fireflies Habitat High-tech Laboratory Estate Lake Miyagase Energy Museum Local museum Aikawa Bridge Suwa Shrine Mimase Takata Hashigiwa Osawa waterfall Prefectural Aikawa Hanbara Elem. Sagami river Ishigoya Dam Community Village Rankaya Hanbara Hanbara shogakko Iriguchi Sumida Kanagawa Central Poultry Farming Association Anzu no Shippo Bakery Battle of Mimase Pass Aikawa Solar Park battlefield marker Takamine Elem. -

Sagamihara Aims to Develop Its Industry and Economy, As Well As to Further Improve Its Shinjuku (Chuo Special Express) 40 Citizens' Quality of Life
Sagami-Ono ⇔ Shinjuku (Rapid Express) Hashimoto Around Easy Access ⇔ Shinjuku JR Yaman (Semi-express) ote Sagamiko 3mins5 L ⇔ i Around ne The city of Sagamihara aims to develop its industry and economy, as well as to further improve its Shinjuku (Chuo Special Express) 40 citizens' quality of life. Around mins Sagamihara City Promotion Map In addition to its existing easy access to major cities around the metropolitan area as well as to 55 mins Shinjuku JR Chuo Main Line *Differ by Haneda Airport, the future extension plan for the Odakyu-Tama Line promises further reinforcement the train service. to the railroad network upon its implementation. JR Chuo Main Line Tokyo J R Also, once the "Linear Chuo Shinkansen", the expected new artery of the nation, starts its operation, SAGAMIHARA Y Chuo Expressway a m access to both Chukyo and Kinki areas will be drastically improved. a n o te A city that leads to the future L in Hachioji IC Odakyu Line e Keio Line 20 Shinagawa Mt. Jinba Hachioji JCT Hachioji Sagamiko-Higashi IC Sagamiko IC Tokyo IC Uenohara IC Takao IC Mt. Takao Sagamiko M Hashimoto Fujino e ( K t r e o n p 412 - O o Sagamihara l Lake Sagami i Linear Chuo Shinkansen E t a Karakida x n Shinagawa Nagoya City p Shin-Yurigaoka ⇔ r I e n Planned to start running in 2027 s t Odakyu-Tama Line s e Sagamihara IC (estimated running time 40 minutes) w r 2027 - Extension Plan a C y ) it y Shinagawa Osaka City JAXA Sagamihara Campus ⇔ Keikyu-Kamata E Kamimizo y x a Planned to start running in 2045 p w (estimated running time 67 minutes) r s 2045 e res s xp Haneda s ei E 413 w Machida om Airport a T y Sagami-Ono J JR Yokohama Line R 129 S a g a 16 Hashimoto Sagamihara m Keikyu Line ⇔ i Aikawa IC L i Sagamihara n Yokohama-Machida IC Around e Shin-Yokohama 3 mins Yokohama Sagamihara City Mt. -

Rising Sun Weekly Email Dispatch
March 19, 2018 + Volume 3, No. 2 SERVING THE U.S. ARMY JAPAN COMMUNITY http://www.facebook.com/USAGJ Rising Sun Weekly Email Dispatch INSIDE THIS VOLUME USAG Japan Social Media Network: Cover: Women's History Month Facebook - http://www.facebook.com/usagj 2 Airbag Recall Vimeo - http://www.vimeo.com/usagj 4 Honoring Vietnam Veterans Twitter - http://www.twitter.com/usagjapan 5 2018 Road Tax Program Flickr - http://www.flickr.com/usagj 6 Read Across America Campaign YouTube - http://www.youtube.com/usagjapan 8 Road Closures Pinterest- http://www.pinterest.com/garrisonjapan/ 9 DFAC Menu Slideshare - http://www.slideshare.net/usagj March 19, 2018 + Volume 3, No. 2 SERVING THE U.S. ARMY JAPAN COMMUNITY http://www.facebook.com/USAGJ Rising Sun Weekly Email Dispatch Safety Alert: Airbag Recall Hot Voting News The manufacturer of Takata airbags is Are you or your friends voting absentee recalling millions of vehicles due to in the Arizona District 8 Special General airbag defects. election? Recall repairs for affected vehicles are You must be registered to vote by March FREE and should be done before May 26, 2018. That's in less than two weeks! 1, 2018 to pass JCI renewal inspections happening on or after this date. Do it now at http://FVAP.gov/Arizona Visit https://www.nhtsa.gov/recall-spotlight/takata-air-bags (ENGLISH) or https:// It's a midterm election year, and you don't www.jaspa.or.jp/portals/recallsearch/index.html (JAPANESE) to determine if your have to wait until November to have your vehicle falls under the recall. -

Program-July 10 Wed
THE 11th INTERNATIONAL SYMPOSIUM ON WATER SUPPLY TECHNOLOGY in YOKOHAMA 2019 Program-July 10 Wed. 5F 503 Session 1 Water Treatment, Water Quality Management and their Future Chair: Yoshihiko Matsui Professor, Graduate School, Hokkaido University Guest Speaker 9:50-10:40 Smart Water System of Seoul: How Smart Is Smart? Young-June Choi General Manager, Bureau of R&D for Water, Office of Waterworks, Seoul Metropolitan Government, South Korea Poster Short Presentation (Details : 14p) 10:50-12:00 Poster Session (Details : 18p) 12:00-13:00 Oral Presentations 13:00-17:35 Construction of a New Water Treatment Plant Combined Magnetic Ion Exchange Resin Process and 13:00 ー 13:20 OP1-1 Advanced Oxidation Process Masahiko Shiba, Ibaraki Prefectural Public Enterprise Bureau Investigation of Introducing Upward Flow Granular Activated Carbon (GAC) Treatment to 13:20 ー 13:40 OP1-2 Nishiya Purification Plant in Yokohama Katsushi Maeda, Kubota Corp. Advanced Treatment by Up-flow Biological Contact Filter in a Full-scale Water Treatment 13:40 ー 14:00 OP1-3 Plant Nguyen Thi Thu Trang, Kobelco-Eco Solutions Co., Ltd. Optimization of Polyaluminum Chloride Dosage with Application of Convolutional Neural 14:00 ー 14:20 OP1-4 Network for Floc Images Eryanti Utami Putri, Chuo University Use of Multi-Disk Stack Filtration (MSF) as Pretreatment for RO Plants 14:20 ー 14:40 OP1-5 Mohammed Alhajji, Saudi Aramco, Saudi Arabia Measures to Prevent Abnormal Water Quality in a Slow Sand Filtration System 14:40 ー 15:00 OP1-6 Suguru Yabashi, Nagoya City Waterworks & Sewerage Bureau 15:00 ー 15:15 Coffee Break UV Disinfection of Water: Current Status and Future Perspectives 15:15 ー 15:35 OP1-7 Kumiko Oguma, The University of Tokyo Development of UV-LED Disinfection Reactor: Design Consideration for Improving 15:35 ー 15:55 OP1-8 Practicability Noriko Igarashi, Swing Engineering Corp. -

Rising Sun Weekly Email Dispatch
March 12, 2018 + Volume 3, No. 1 SERVING THE U.S. ARMY JAPAN COMMUNITY http://www.facebook.com/USAGJ Rising Sun Weekly Email Dispatch INSIDE THIS VOLUME USAG Japan Social Media Network: C Easter Egg Hunt Facebook - http://www.facebook.com/usagj 2 Local Nationals Town Hall Vimeo - http://www.vimeo.com/usagj 4 Lent-Easter Events 2018 Twitter - http://www.twitter.com/usagjapan 5 2018 Road Tax Program Flickr - http://www.flickr.com/usagj 6 Read Across America Campaign YouTube - http://www.youtube.com/usagjapan 7 MWR March Calendar Pinterest- http://www.pinterest.com/garrisonjapan/ 9 DFAC Menu Slideshare - http://www.slideshare.net/usagj March 12, 2018 + Volume 3, No. 1 SERVING THE U.S. ARMY JAPAN COMMUNITY http://www.facebook.com/USAGJ Rising Sun Weekly Email Dispatch USAJOBS Adopts New Login Hot Voting News USAJOBS has adopted Login.Gov as a platform to make the federal application As we begin the month of March, we also process easier, more secure. What does this begin the 2018 Election Season. change mean to your USAJOBS account? As of March 6, 2018 there are 16 different States holding Primaries or Special Elections over the next 90 days in preparation for the Nothing will happen to the information Nov. 6, 2018 General Election. already stored in your USAJOBS account and profile. You will be able to keep all of your Please check https://www.fvap.gov/guide/ applications as well saved searches and job upcoming-elections to see if your State is announcements. one of them. Once you set up a login.gov account, you will connect the account to your USAJOBS If you requested your absentee ballot and profile. -
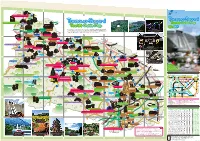
Open to the Public
A B C D E F 521 Wada no Sato Experience Center "Village House" G H Mt. Jimba Tanzawa-Sagami 100 Japanese Rural Villages・ Sanogawa Area To Hachioji Tanzawa Sagami Jimba no Yu - Tourist Guide Map 522 1 Touristst GuGuiddee Mapp 1 たび相模 Ishii House 旅 丹沢・相模 観光ナビ Expresswa uo y Ch Sagamiko-Higashi Kanagawa Prefectural Government Website: Tanzawa-Sagami Tourist Guide To Kofu Obarajuku Honjin Midori's Love Letter Lake Tsukui Ogura Bridge Sagamiko IC IC provides sightseeing information: places to visit, souvenirs to purchase, and Midori Ward Sagamihara City (Fujino area) Midori Ward Sagamihara City (Tsukui area) Midori Ward Sagamihara City (Shiroyama area) K MAP - MAP - MAP - osh A 2 /D 2 Guide: Nature /E 2 Guide: History u Higway e in . It's updated regularly. Check it out online! L 20 To Takao events to experience 20 uo Ch Fujino JR Sagamiko Lake Sagami Yoshino-juku Fujiya Sagamiko Memorial Hall Prefectural Sagamiko Park To Hachioji Lake Shiroyama Shiroyama lake promenade To Hachioji To Hachioji Art Walk Sagamiko Resort Pleasure Forest Shokakuji Temple 515 Sagamihara City Mt.Sekiro To Chofu Ozaki Gakudo Memorial House Midori Ward Lake Tsukui 413 (Shiroyama Area) Hashimoto e Fujino Hot Springs Higashiotaru no Yu Lin ihara gam io Sa 2 Prefectural Fujino Art House Sagamihara City 412 Sagamihara City Ke 2 517 J Midori Ward Midori Ward R Y ok Obarajuku Honjin Shinohara no Sato Center oh (Sagamiko Area) am a Midori Ward Sagamihara City (Sagamiko area) Lin Ogura Bridge e /MAP C-1 Guide: History Fujino Yamanami Hot Springs IC Sagamihara Sagamigawa Nature Village Park 518 Sagamihara Prefectural Tsukuiko-Shiroyama Park 508 Minami- 129 Hashimoto Sagamihara City Yabe Kami-Ooshima Campgrounds Midori Ward 16 Doshi Riv. -

Data of Geochemistry
Data of Geochemistry * Chapter G. Chemical Composition of Rivers and Lakes GEOLOGICAL SURVEY PROFESSIONAL PAPER 440-G Data of Geochemistry MICHAEL FLEISCHER, Technical Editor Chapter G. Chemical Composition of Rivers and Lakes By DANIEL A. LIVINGSTONE GEOLOGICAL SURVEY PROFESSIONAL PAPER 440-G UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1963 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. DATA OF GEOCHEMISTRY, SIXTH EDITION Michael Fleischer, Technical Editor The first edition of the Data of Geochemistry, by F. W. Clarke, was published in 1908 as U.S. Geological Survey Bulletin 330. Later editions, also by Clarke, were published in 1911, 1916, 1920, and 1924 as Bulletins 491, 616, 695, and 770. This, the sixth edition, has been written by several scientists in the Geological Survey and in other institutions in the United States and abroad, each preparing a chapter on his special field. The current edition is being published in individual chapters, titles of which are listed below. Chapters already published are indicated by boldface. CHAPTER A. The chemical elements B. Cosmochemistry C. Internal structure and composition of the Earth D. Composition of the earth's crust E. Chemistry of the atomsphere F. Chemical composition of subsurface waters, by Donald E. White, John D. Hem, and G. A. Waring G. Chemical composition of rivers and lakes, by Daniel A. Livingstone H. Chemistry of the oceans I. Geochemistry of the biosphere J. Chemistry of rock-forming minerals K. Volcanic emanations, by Donald E. -

Tourist Attraction Sheets Can Be Downloaded Here
Tourist Attraction Sheets can be downloaded here http://www.pref.kanagawa.jp/ Tourist Attraction Sheets docs/b6m/conference/index.html NO Theme Area City Main Content Sub Content① Sub Content② 1 History Yokohama・Kawasaki Yokohama Sail Training Ship NIPPONMARU Yokohama Port Museum Minato Mirai Deck Cleaning 2 Landscape Yokohama・Kawasaki Yokohama Yokohama Sky Cruise Yokohama Port Cruising Marine Rouge Minato Mirai 3 Landscape Yokosuka・Miura Area Miura Riviera Seabonia Marina Forest of Koajiro Yacht gramping 4 Landscape Yokosuka・Miura Area Yokosuka Yokosuka Naval Port Cruise Dobuita Street Jogashima 5 Landscape Yokosuka・Miura Area Yokosuka Sarushima Soleil no Oka Strawberry picking Chigasaki Southern C / 6 Landscape Shonan Area Chigasaki Eboshi Rock Cruise Hamaori Festival Headland Boardwalk 7 History Yokohama・Kawasaki Yokohama Shomyoji Temple Yokohama Hakkejima Sea Paradise Ito Hirobumi Old Villa in Kanazawa 8 History Yokohama・Kawasaki Yokohama Sotoshu Daihonzan Sojiji Temple Kirin Yokohama Beer Village Namamugi Fish Market 9 History Yokohama・Kawasaki Yokohama Sankeien Garden Try Matcha Green Tea Unique Venue 10 History Yokohama・Kawasaki Yokohama Tour of Yamatenishi Western-style Buildings Motomachi Shopping Street Yamashita Park 11 History Yokosuka・Miura Area Kamakura Flower Tour of Kamakura Kamakura Museum of Literature Hasedera Temple Zuisenji Temple 12 History Western Area Minamiashigara Daiyuzan Saijoji Temple Modern Bathing - Only You Odawara Castle 13 Shopping Arcade Yokohama・Kawasaki Yokohama Yokohama Kofuku-ji Matsubara Shopping -

Sagamiko Illumillion (Sagamiko Resort Pleasure Forest)
Kanagawa sightseeing charm creation conference Central of <Amusement> the Prefecture Sagamihara City Bodily-sensational Illumination: Wrapped in light Tourist Attraction No. 1871 Sagamiko Illumillion (Sagamiko Resort Pleasure Forest) Sagamiko Illumillion is the biggest illumination event in the Kanto region held every year by Sagamiko Resort. This experience-type illumination, which features 6 million beautiful balls of light, is recognized Explanation of as one of the big three illumination events of Kanto. The place is 50 minutes by car from the center of Tourist Attraction the Metropolis, and because it has various facilities such as an amusement park, camp and barbecue grounds, and bathing facilities for day trips, anyone and everyone can enjoy themselves. There are also plenty of night attractions such as lifts and a Ferris wheel. You can enjoy a selling point mystical experience in which your body is wrapped by the overwhelming radiance of Kanto’s largest decorative illumination event. Address 1634 Wakayanagi, Midori-ku, Sagamihara-shi, Kanagawa-ken Opening Hours From early November to early April The time that the illuminations are lit vary seasonally. For details, please check the website. Availability of Parking Available (regular vehicle \1,000, Large bus \2,000, Two wheeled vehicles \500) URL https://www.sagamiko-resort.jp/events/illumillion.html(Website is available in multiple languages.) Recommended Season November-April Access Group/Individual Mark Group Individual From Sagamiko Station (JR Line) take a Bus bound for Mikage. Get off at Pleasure Target Regions Europe, North America, Oceania, Asia Forest-mae bus stop and walk for 1 min. There is a nonstop bus service from Shinjuku Station (charges apply). -

Climatic Zone: Subjects: Effects: Project Name: Country
IEA Hydropower Implementing Agreement Annex VIII - Hydropower Good Practices: Environmental Mitigation Measures and Benefits Case Study 14-03: Development of Regional Industries – Miyagase Dam, Japan Key Issues: 14- Development of Regional Industries 1-Biological Diversity 7-Resttlement Climatic Zone: Cf : Temperate Humid Climate Subjects: - Development of Sightseeing and Other Facilities to Vitalize the Area Around the Dam Effects: - Nature preservation - Stimulation of local industries through development of sightseeing spots Project Name: Miyagase Dam Country ::: Kanagawa Prefecture, Japan(Asia) Implementing Party & Period - Project: the Ministry of Land, Infrastructure and Transport 1987.11 through 2001.4 - Good Practice: the Ministry of Land, Infrastructure and Transport 2001.4 - Keywords: Environmental protection, biotope, stimulation of local development, vitalization of the water source area, tourism development Abstract ::: Activities, including the development of biotopes, were conducted to restore the natural environment in the area impacted by the dam development project. In addition, activities to develop sightseeing facilities such as an information center were conducted after completion of the dam to stimulate local development. The number of tourists visiting Kiyokawa Village, where the dam had been constructed, and the total tourist spending increased more than five times compared to the figures before the implementation of the project. (See Fig. 4) 1. Outline of the Project The Miyagase Dam is a concrete gravity dam with a height of 156 m, a volume of approximately 2 million m 3 and a gross storage capacity of approximately 200 million m 3. It was constructed on the Nakatsu River, which is a tributary of the Sagami River, a first-class river originating in the Tanzawa Mountain Range located in the western part of Kanagawa Prefecture, to serve the multiple purposes of flood control, river environment improvement, city water supply and power generation. -

Parkark (Outbound Lane Only from Tokyo) Sagamiko Lakefront Aprilapril 20 Cherry Festival Chuo Expressway
SagamikoIC to Takao Shinjuku AnnualAnnual EventEvent ScheduleSchedule Sagamiko East Exit LLakeake SSagamiagami PPrefecturalrefectural PParkark (Outbound lane only from Tokyo) Sagamiko Lakefront AprilApril 20 Cherry Festival Chuo Expressway Yose Shrine Grand Festival 20 JRJR Chuo Main Line Chu o M Yamanami Festival ain iver Lin e JR Sagamiko Station Sagami R MayMay Culture Association Festival Sagamiko Sagami Dam Shokakuji Temple Azalea 20 Waterfall blooms to Otsuki LakeLake SagamiSagami ParkPark BusBus StopStop Lake Sagami Bridge LakeLake JulyJuly Sagamiko Dam Festival 県Lake 立 AugustAugust Lake Sagami 412 SSagamiagami Mt. Arashi Fireworks Festival 相模湖相Sagami模 湖 Sagamiko Lakeside Prefectural Beer Garden 公園公Park園 SeptemberSeptember PPrefecturalrefectural LA I Stunning fireworks at the Lake Sagami Citizens Regatta K E S A G A M Fireworks Festival. ttoo KKen-Oen-O EExpresswayxpressway SSagamiharaagamihara IInterchangenterchange OctoberOctober Lake Sagami ParkPark Community Fair Address Lake Sagami Regatta NovemberNovember Kanagawa-ken,Sagamihara-shi, Midori-ku,Yose 317 post L A I Obara Inn Festival code 252-0171 K E S A G A M Lake Sagami Hometown Festival Access by train DecemberDecember Lake Sagami Yamanami ・ 10 minute walk from JR Chuo line’s Sagamiko Station Illumination JanuaryJanuary Sagamiko Long Distance Access by bus Relay Race ・ Take the Mikage bound bus from JR Yokohama & Keio Line’s FebruaryFebruaryYose Shrine Setsubun Festival Hashimoto Station - 30 min. plus Change at Mikage bus depot Kanagawa Long for a bus bound for Sagamiko Station, get off at Lake Sagami Distance Relay Race Park bus stop - 15 min. MarchMarch Obara Inn Illumination Akiba Shrine Fire Festival Access by car ・ A 2km drive after exiting Chuo expressway’s Sagamiko East Exit (outbound traffic only from Tokyo) or a 4 km drive after exiting Sagamiko Interchange.