Apheresis Red Cell Exchange/Transfusions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood, Lymph, and Immunity Joann Colville
Blood, Lymph, and Immunity Joann Colville CHAPTER OUTLINE .»: BLOOD Function Introduction Lymphatic Structures Plasma THE IMMUNE SYSTEM Cellular Components of Blood Function Red Blood Cells Immune Reactions Platelets Immunization: Protection Against Disease White Blood Cells THE LYMPHATICSYSTEM Lymph Formation Characteristics LEARNING OBJECTIVES • List and describe the functions of blood • List the functions of the lymphatic system • Describe the composition of blood plasma • Describe the structure and functions of the lymph nodes, • Describe the characteristics of mature erythrocytes spleen, thymus, tonsils, and GALT Describe the structure of the hemoglobin molecule and • List the functions of the immune system explain the fate of hemoglobin following intravascular and • Differentiate between specific and nonspecific immune extravascular hemolysis reactions • Give the origin of thrombocytes and describe their • Differentiate between cell-mediated and humoral immunity characteristics and functions • List the components involved in cell-mediated immunity • Listthe types of leukocytes and describe the functions of each and explain the role of each • Describe the formation of lymph fluid and its circulation List and describe the classes of immunoglobulins through the lymphatic system • Differentiate between active and passive immunity BLOOD vessels of the cardiovascular system. It has three main func- tions: transportation, regulation, and defense. INTRODUCTION Blood. Say the word, and some people cringe, others faint, Function and if you believe authors Bram Stoker, Stephen King, and 1. Blood is a transport system. Christopher Moore (all authors of vampire books), others • It carries oxygen, nutrients, and other essential com- drool. But no matter what you think about blood, animals pounds to every living cell in the body. -

The Diagnosis of Paroxysmal Nocturnal Hemoglobinuria: Role of Flow Cytometry
THE DIAGNOSIS OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: ROLE OF FLOW CYTOMETRY Alberto Orfao Department of Medicine, Cancer Research Centre (IBMCC-CSIC/USAL), University of Salamanca, Salamanca, Spain Paroxysmal nocturnal hemoglobinuria (PNH) on one or multiple populations of peripheral blood is an acquired clonal disorder typically affecting red cells and/or leucocytes of PNH patients, this young adults, which involves the phosphatidylino- translating into a new diagnostic test. Because sitol glycan anchor biosynthesis class A gene of this flow cytometry became an essential tool (PIGA) coded in chromosome X, in virtually every in the diagnosis of PNH. In turn, it could be also case. The altered gene encodes for a defective pro- demonstrated that investigation of the presence of tein product, the pig-a enzyme which is involved GPI-deficient cells among circulating mature neu- in the early steps of the synthesis of glycosylphos- trophils and monocytes, increases the sensitivity phatidylinositol (GPI). This later molecule (GPI) of red blood cell screening because of the shorter acts as an anchor of a wide range of proteins to lifetime of both populations of leucocytes. Despite the cell surface. At present a large number of GPI- all the above, routine assessment of CD55 and anchor associated proteins have been described CD59 is also associated with several limitations which are expressed by one or multiple distinct due to distinct patterns of expression among dif- compartments of hematopoietic cells. The altered ferent cell populations, dim and heterogeneous PIGA gene leads to an altered GPI anchor which expression among normal individuals and the lack leads to defective expression of GPI-AP on the of experience in many labs as regards the normal cytoplasmic membrane, on the cell surface. -

Policy and Procedure
Policy and Procedure Title: Exchange Transfusion for Sickle Cell Division: Medical Management Disease Department: Utilization Management Approval Date: 2/9/18 LOB: Medicaid, Medicare, HIV SNP, CHP, MetroPlus Gold, Goldcare I&II, Market Plus, Essential, HARP Effective Date: 2/9/18 Policy Number: UM-MP224 Review Date: 1/18/19 Cross Reference Number: Retired Date: Page 1 of 7 1. POLICY: Exchange Transfusion for Sickle Cell Disease 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Claims Department, Provider Contracting 3. DEFINITIONS • Sickle cell disease – Sickle cell disease (SCD) refers to a group of inherited disorders characterized by sickled red blood cells (RBCs), caused either by homozygosity for the sickle hemoglobin mutation (HbSS; sickle cell anemia) or by compound heterozygosity for the sickle mutation and a second beta globin gene mutation (e.g., sickle-beta thalassemia, HbSC disease). In either HbSS or compound heterozygotes, the majority of Hgb is sickle Hgb (HgbS; i.e., >50 percent). • Transfusion – Simple transfusion refers to transfusion of RBCs without removal of the patient's blood. • Exchange Transfusion – Exchange transfusion involves transfusion of RBCs together with removal of the patient's blood. Exchange transfusion can be performed manually or via apheresis (also called cytapheresis or hemapheresis) using an extracorporeal continuous flow device. 4. PROCEDURE: A. Exchange transfusion for sickle cell disease will be covered as an ambulatory surgery procedure when all the following criteria are met: i) The member has documented SCD. ii) The exchange transfusion is a pre-scheduled procedure. iii) The purpose of the exchange transfusion is to prevent stroke, acute chest syndrome, or recurrent painful episodes. -

Sickle Cell Disease: Chronic Blood Transfusions
Sickle Cell Disease: Chronic Blood Transfusions There may be times when sickle cell patients require a blood transfusion. Such situations include preparing for surgery, during pregnancy, or during a severe complication such as an aplastic crisis, splenic sequestration or acute chest syndrome. In these cases, transfusion is a one-time intervention used to reduce the severity of the complication you are experiencing. However, if you have had a stroke, or an MRI or TCD shows that you are at high risk for having a stroke, your hematologist may recommend you begin chronic blood transfusions. What Does a Blood Transfusion Do? What are The Risks? Chronic (monthly) blood transfusions have been proven to Blood transfusions are not without risks. One risk is drastically reduce a sickle cell patient’s risk of stroke. They alloimmunization, a process in which the patient receiving have also been shown to reduce the frequency, severity blood transfusions creates antibodies to certain types of and duration of other sickle cell complications. Sickle cell blood. As a result he/she may have a reaction to the blood patients usually have a hemoglobin S level of about 80- that was transfused. Alloimmunization makes it more 90%. This means 80-90% of the circulating red blood cells difficult to find blood that is a good match for the patient. are cells that can sickle and cause complications. The goal In order to prevent alloimmunization, some centers of chronic blood transfusion therapy is to bring that routinely perform RBC phenotyping (special testing for percentage down below 30%. This will mean fewer sickle antibodies) on sickle cell disease patients so that they may cells circulating in the body, and a lower risk of give blood that is a better match for the patient. -

Chapter 06 Lecture Outline
Chapter 06 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © 2016 McGraw-Hill Education. Permission required for reproduction or display. 1 Cardiovascular System: Blood © SPL/Science Source, (inset) © Andrew Syred/Science Source 2 Points to ponder • What type of tissue is blood and what are its components? • What is found in plasma? • Name the three formed elements in blood and their functions. • How does the structure of red blood cells relate to their function? • Describe the structure and function of each white blood cell. • What are disorders of red blood cells, white blood cells, and platelets? • What do you need to know before donating blood? • What are antigens and antibodies? • How are ABO blood types determined? • What blood types are compatible for blood transfusions? • What is the Rh factor and how is this important to pregnancy? • How does the cardiovascular system interact with other systems to maintain homeostasis? 3 6.1 Blood: An Overview What are the functions of blood? • Transportation: oxygen, nutrients, wastes, carbon dioxide, and hormones • Defense: against invasion by pathogens • Regulatory functions: body temperature, water- salt balance, and body pH 4 6.1 Blood: An Overview What is the composition of blood? • Remember: blood is a fluid connective tissue. • Formed elements are produced in red bone marrow. – Red blood cells/erythrocytes (RBCs) – White blood cells/leukocytes (WBCs) – Platelets/thrombocytes 5 6.1 Blood: An Overview What is the composition of blood? • Plasma – It consists of 91% water and 9% salts (ions) and organic molecules. – Plasma proteins are the most abundant organic molecules. -

Pediatric Orthotopic Heart Transplant Requiring Perioperative Exchange Transfusion: a Case Report
JECT. 2004;36:361–363 The Journal of The American Society of Extra-Corporeal Technology Case Reports Pediatric Orthotopic Heart Transplant Requiring Perioperative Exchange Transfusion: A Case Report Brian McNeer, BS; Brent Dickason, BS, RRT; Scott Niles, BA, CCP; Jay Ploessl, CCP The University of Iowa Hospitals and Clinics, Iowa City, Iowa Presented at the 41st International Conference of the American Society of Extra-Corporeal Technology, Las Vegas, Nevada, March 6–9, 2003 Abstract: An 11-month-old patient with idiopathic cardio- the venous line just proximal to the venous reservoir while si- myopathy was scheduled for orthotopic heart transplantation. A multaneously transfusing the normalized prime at normother- perioperative exchange transfusion was performed because of mia. Approximately 125% of the patients calculated blood vol- elevated panel reactive antibody levels. This process was accom- ume was exchanged. This technique greatly reduces the likeli- plished in the operating room prior to instituting cardiopulmo- hood of hyperacute rejection. The exchange transfusion process, nary bypass using a modified cardiopulmonary bypass circuit. In in addition to the patient immature immune system, provides preparation for the procedure, the cardiopulmonary bypass cir- additional options in orthotopic heart transplantation for pa- cuit was primed with washed leukocyte-filtered banked packed tients that may otherwise not be considered suitable candi- red blood cells, fresh-frozen plasma, albumin, and heparin. Pump dates. Keywords: exchange transfusion, heart transplant, pediat- prime laboratory values were normalized prior to beginning the ric, panel reactive antibodies. JECT. 2004;36:361–363 exchange transfusion. The patient’s blood was downloaded from Despite continuing advances in the management of end- humoral sensitization is determined by the presence of a stage cardiac failure, cardiac transplantation remains the positive panel reactive antibody (PRA) screen. -
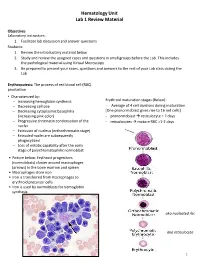
Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses. -
Laboratory Best Transfusion Practice for Neonates, Infants and Children
Laboratory Best Transfusion Practice for Neonates, Infants and Children This summary guidance should be used in conjunction with the appropriate 20161 and 20122 BSH Guidelines and laboratory SOPs Compatibility testing Neonates and infants < 4 months Obtain neonatal and maternal transfusion history (including any fetal transfusions) for all admissions. Obtain a maternal sample for initial testing where possible, in addition to the patient sample. Red cell selection: no maternal antibodies present Select appropriate group and correct neonatal specification red cells. Group O D-negative red cells may be issued electronically without serological crossmatch. If the laboratory does not universally select group O D-negative red cells for this age group, blood group selection should either be controlled by the LIMS or an IAT crossmatch should be performed using maternal or neonatal plasma to serologically confirm ABO compatibility with both mother and neonate. Red cell selection: where there is maternal antibody Select appropriate group red cells, compatible with maternal alloantibody/ies. An IAT crossmatch should be performed using the maternal plasma. If it is not possible to obtain a maternal sample it is acceptable to crossmatch antigen-negative units against the infant’s plasma. Where paedipacks are being issued from one donor unit it is only necessary to crossmatch the first split pack. Subsequent split packs from this multi-satellite unit can be automatically issued without further crossmatch until the unit expires or the infant is older than 4 months. If packs from a different donor are required, an IAT crossmatch should be performed. Infants and children ≥ 4 months For infants and children from 4 months of age, pre-transfusion testing and compatibility procedures should be performed as recommended for adults. -

SEED Haematology Sysmex Educational Enhancement and Development October 2012
SEED Haematology Sysmex Educational Enhancement and Development October 2012 The red blood cell indices The full blood count has been used in conjunction with the traditional red The complete blood count (CBC) is central to clinical deci- cell indices in order to narrow down the possible causes sion making. This makes it one of the commonest laboratory of anaemia in an individual patient. investigations performed worldwide. Whilst the definition of what constitutes an CBC is influenced by the number Impedance technology and type of parameters measured by different haematology The RBC, HCT and MCV are all closely interrelated as they analysers, the traditional red cell indices that are widely are derived from information obtained from the passage used to classify anaemias are common to all. of cells through the aperture of the impedance channel of an automated haematology analyser. The impedance The laboratory approach to anaemia technology is based on the principle that an electrical field, Anaemia is an extremely common global healthcare prob- created between two electrodes of opposite charge, can lem. However, anaemia is merely a symptom which can be used to count and determine the size of cells. Blood result from a multitude of causes. Effective treatment is cells are poor conductors of electricity. The diluent in which only possible if the underlying cause is correctly identified. they are suspended as they pass through the aperture To this end, several classification systems have been devis- during counting is an isotonic solution which is a good ed. The most useful and widely used classification system conductor of electricity. -

Indicators of the Iron Status of Populations: Red Blood Cell Parameters Sean Lynch
AnneX 1 Indicators of the iron status of populations: red blood cell parameters SEAN LYNch 19 Indicators OF the iron statUS OF popUlations: red blood cell parameters Contents 1. Introduction 22 2. Relationship between anaemia and iron deficiency 23 2.1 Physiological control of haemoglobin levels 23 2.2 Relationship between iron deficiency and anaemia 24 3. Red blood cell parameters 26 3.1 Haemoglobin 26 3.2 Hematocrit or packed cell volume 26 3.3 Mean cell volume and mean cell haemoglobin 27 3.4 Red cell distribution width 27 3.5 Reticulocyte haemoglobin concentration and percentage of hypochromic red cells 27 4. Role of haemoglobin as a screening indicator for iron deficiency 27 5. Conclusions 28 6. References 29 21 Assessing the iron statUS OF popUlations 1. Introduction Iron is an essential nutrient that plays a central role in many metabolic processes. Aerobic metabolism is critically dependent on maintaining normal concentrations of several iron-containing proteins that mediate oxygen transport, storage and utili- zation, particularly when the tissue demand for oxygen is increased by physical ac- tivity. Pioneering research over the last 50 years, much of it stemming from concepts developed and validated experimentally by Dr Clement Finch and his coworkers, led to the recognition that a negative iron balance resulting from an iron intake insuf- ficient to match losses from the body despite compensatory changes in the rate of absorption and, to a more limited extent, excretion could be divided into three stages based on the severity of the potential effect on physiological functions. The evalua- tion of functional impairment was related entirely to erythropoiesis for two reasons: the effects of changes in iron status on blood elements are readily evaluated, while the effect on the enzymes in other tissues necessitates obtaining biopsy samples. -

Use of Blood Components in the Newborn
NNF Clinical Practice Guidelines Use of Blood Components in the Newborn Summary of recommendations • Transfusion in the newborn requires selection of appropriate donor, measures to minimize donor exposure and prevent graft versus host disease and transmission of Cytomegalovirus. • Component therapy rather than whole blood transfusion, is appropriate in most situations. • A clear cut policy of cut-offs for transfusions in different situations helps reduce unnecessary exposure to blood products. • Transfusion triggers should be based on underlying disease, age and general condition of the neonate. Writing Group : Chairperson: Arvind Saili ; Members: RG Holla, S Suresh Kumar Reviewers: Neelam Marwaha, Ruchi Nanawati Page | 129 Downloaded from www.nnfpublication.org NNF Clinical Practice Guidelines Introduction Blood forms an important part of the therapeutic armamentarium of the neonatologist. Very small premature neonates are amongst the most common of all patient groups to receive extensive transfusions. The risks of blood transfusion in today’s age of rigid blood banking laws, while infrequent, are not trivial. Therefore, as with any therapy used in the newborn, it is essential that one considers the risk- benefit ratio and strive to develop treatment strategies that will result in the best patient outcomes. In addition, the relatively immature immune status of the neonate predisposes them to Graft versus Host Disease (GVHD), in addition to other complications including transmission of infections, oxidant damage, allo- immunization and