Biological Control in Plant Protection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final Report
Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final report Dr. Iryna Matsiakh Tbilisi 2014 This publication has been produced with the assistance of the European Union. The content, findings, interpretations, and conclusions of this publication are the sole responsibility of the FLEG II (ENPI East) Programme Team (www.enpi-fleg.org) and can in no way be taken to reflect the views of the European Union. The views expressed do not necessarily reflect those of the Implementing Organizations. CONTENTS LIST OF TABLES AND FIGURES ............................................................................................................................. 3 ABBREVIATIONS AND ACRONYMS ...................................................................................................................... 6 EXECUTIVE SUMMARY .............................................................................................................................................. 7 Background information ...................................................................................................................................... 7 Literature review ...................................................................................................................................................... 7 Methodology ................................................................................................................................................................. 8 Results and Discussion .......................................................................................................................................... -

The Compositional and Configurational Heterogeneity of Matrix Habitats Shape Woodland Carabid Communities in Wooded-Agricultural Landscapes
The compositional and configurational heterogeneity of matrix habitats shape woodland carabid communities in wooded-agricultural landscapes Article Accepted Version Neumann, J. L., Griffiths, G. H., Hoodless, A. and Holloway, G. J. (2016) The compositional and configurational heterogeneity of matrix habitats shape woodland carabid communities in wooded-agricultural landscapes. Landscape Ecology, 31 (2). pp. 301-315. ISSN 0921-2973 doi: https://doi.org/10.1007/s10980-015-0244-y Available at http://centaur.reading.ac.uk/46912/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1007/s10980-015-0244-y Publisher: Springer All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online The compositional and configurational heterogeneity of matrix habitats shape woodland carabid communities in wooded- agricultural landscapes. Jessica L. Neumann1,3*, Geoffrey H. Griffiths1, Andrew Hoodless2 and Graham J. Holloway3 1 Department of Geography and Environmental Science, University of Reading, Reading, UK 2 Game and Wildlife Conservation Trust, Fordingbridge, Hampshire, UK 3 Centre for Wildlife Assessment and Conservation, Department of Biological Sciences, University of Reading, Reading, UK Abstract Context Landscape heterogeneity (the composition and configuration of matrix habitats) plays a major role in shaping species communities in wooded-agricultural landscapes. -
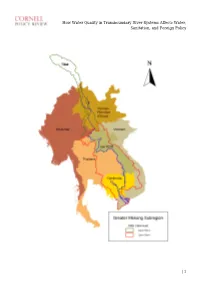
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy | 1 How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy David Tipping, 2001 By David C. Tipping Edited by Yeareen Yun Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any agency of the Australian government. Assumptions made within the analysis are not reflective of the position of any Australian government entity, or other organization or professional association. 1. INTRODUCTION Access to adequate water supply and sanitation is the core premise of local level water security. Effective management of transboundary river basin systems and water quality risks is therefore fundamental to social progress and quality of life. Improved water quality management benefits many individual lives in riparian nations, and, as demonstrated by the annual new year blessing of the fish migrations, society at large throughout the Mekong River Basin. In 2001, the author investigated the use of sustainable development indicators to improve the institutional effectiveness of international environmental management regimes. A new framework was designed to evaluate beneficial uses of water. In addition, a case study was developed on the Lower Mekong River Basin system, which integrated measures of water and environmental quality and socio-economic development. The research objectives were: (1) improving the understanding of water quality issues; (2) benchmarking water resources management performance at local, national and regional levels; and (3) enhancing technical and administrative capabilities of transboundary river basin management regimes through capacity development focused on the achievement of sustainable development objectives, and obligations and duties under international law. -

Field Release of the Insects Calophya Latiforceps
United States Department of Field Release of the Insects Agriculture Calophya latiforceps Marketing and Regulatory (Hemiptera: Calophyidae) and Programs Pseudophilothrips ichini Animal and Plant Health Inspection (Thysanoptera: Service Phlaeothripidae) for Classical Biological Control of Brazilian Peppertree in the Contiguous United States Environmental Assessment, May 2019 Field Release of the Insects Calophya latiforceps (Hemiptera: Calophyidae) and Pseudophilothrips ichini (Thysanoptera: Phlaeothripidae) for Classical Biological Control of Brazilian Peppertree in the Contiguous United States Environmental Assessment, May 2019 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. -

Cecidophyopsis Ribis Westw.)
Danish Research Service for Plant and Soil Science Report no. 1880 Research Centre for Plant Proteetion Institute of Pesticides D K-2800 Lyngby Chemicais test e d in the laboratory for the control of ofblack currant gall mite (Cecidophyopsis ribis Westw.) Afprøvning i laboratoriet af bekæmpelsesmidlers virkning mod solbærknopgalmider (Cecidophyopsis ribis Westw.) Steen ~kke Nielsen Summary 32 chemicals were teste d in the laboratory against the black currant gall mite (Cecidophyopsis ribis Westw.). Only 5 chemicals showed an acceptable controlling effect. They were endosulfan, oxamyl, avermectin, lime sulphur and wettable sulphur. 8 pyrethroids, 3 other insecticides and 8 acaricides were all ineffective and the same applied to 8 fun gicides normally used against diseases in black currants. Key words: Chemicals, laboratory test, black currant gal! mite, Cecidophyopsis ribis Westw. Resume 32 pesticider blev afprøvet i laboratoriet for deres virkning mod solbærknopgalmider (Cecidophyopsis ribis Westw.). Kun 5 midler gaven tilfredsstillende virkning. Det var endosulfan, oxamyl, avermectin, svovlkalk og sprøjtesvovl. 8 pyrethroider, 3 andre insekticider og 8 acaricider havde ingen eller en util strækkelig virkning mod solbærknopgalmiderne. Det samme gjaldt for 8 fungicider, der er almindeligt benyttet til bekæmpelse af svampesygdomme i solbær. Nøgleord: Pesticider, laboratorie-test, solbærknopgalmider, Cecidophyopsis ribis Westw. Introduction ternatives. Different types of chemicals were cho The most serious pest in black currants in Den sen to be tested for their con tro Iling effect on the mark is the black currant gall mite. Black currants gall mite: Pyrethroids because of their low mam are a rather small crop, so little effort has been malian toxicity and their repellent action against made to find suitable chemicals to control the gall honeybees; acaricides with harvest intervals of 4 mite. -

Farming System and Habitat Structure Effects on Rove Beetles (Coleoptera: Staphylinidae) Assembly in Central European Apple
Biologia 64/2: 343—349, 2009 Section Zoology DOI: 10.2478/s11756-009-0045-3 Farming system and habitat structure effects on rove beetles (Coleoptera: Staphylinidae) assembly in Central European apple and pear orchards Adalbert Balog1,2,ViktorMarkó2 & Attila Imre1 1Sapientia University, Faculty of Technical Science, Department of Horticulture, 1/C Sighisoarei st. Tg. Mures, RO-540485, Romania; e-mail: [email protected] 2Corvinus University Budapest, Faculty of Horticultural Science, Department of Entomology, 29–43 Villányi st., A/II., H-1118 Budapest, Hungary Abstract: In field experiments over a period of five years the effects of farming systems and habitat structure were in- vestigated on staphylinid assembly in Central European apple and pear orchards. The investigated farms were placed in three different geographical regions with different environmental conditions (agricultural lowland environment, regularly flooded area and woodland area of medium height mountains). During the survey, a total number of 6,706 individuals belonging to 247 species were collected with pitfall traps. The most common species were: Dinaraea angustula, Omalium caesum, Drusilla canaliculata, Oxypoda abdominale, Philonthus nitidulus, Dexiogya corticina, Xantholinus linearis, X. lon- giventris, Aleochara bipustulata, Mocyta orbata, Oligota pumilio, Platydracus stercorarius, Olophrum assimile, Tachyporus hypnorum, T. nitidulus and Ocypus olens. The most characteristic species in conventionally treated orchards with sandy soil were: Philonthuss nitidulus, Tachyporus hypnorum, and Mocyta orbata, while species to be found in the same regions, but frequent in abandoned orchards as well were: Omalium caesum, Oxypoda abdominale, Xantholinus linearis and Drusilla canaliculata.ThespeciesDinaraea angustula, Oligota pumilio, Dexiogya corticina, Xantholinus longiventris, Tachyporus nitidulus and Ocypus olens have a different level of preferences towards the conventionally treated orchards in clay soil. -

Efficacy and Host Specificity Compared Between Two Populations of The
Biological Control 65 (2013) 53–62 Contents lists available at SciVerse ScienceDirect Biological Control journal homepage: www.elsevier.com/locate/ybcon Efficacy and host specificity compared between two populations of the psyllid Aphalara itadori, candidates for biological control of invasive knotweeds in North America ⇑ Fritzi Grevstad a, , Richard Shaw b, Robert Bourchier c, Paolo Sanguankeo d, Ghislaine Cortat e, Richard C. Reardon f a Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR 97331, USA b CABI, Bakeham Lane, Egham, Surrey TW20 9TY, United Kingdom c Agriculture and AgriFood Canada-Lethbridge Research Centre, Lethbridge, AB, Canada T1J 4B1 d Olympic Natural Resources Center, University of Washington, Forks, WA 98331, USA e CABI, CH 2800 Delemont, Switzerland f USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, WV 26505, USA highlights graphical abstract " Two populations of the psyllid Aphalara itadori are effective at reducing knotweed growth and biomass. " The two populations differ in their performance among different knotweed species. " Development of A. itadori occurred infrequently on several non-target plant species. " The psyllid exhibited non-preference and an inability to persist on non- target plants. article info abstract Article history: Invasive knotweeds are large perennial herbs in the Polygonaceae in the genus Fallopia that are native to Received 2 February 2012 Asia and invasive in North America. They include Fallopia japonica (Japanese knotweed), F. sachalinensis Accepted 4 January 2013 (giant knotweed), and a hybrid species F. x bohemica (Bohemian knotweed). Widespread throughout Available online 12 January 2013 the continent and difficult to control by mechanical or chemical methods, these plants are good targets for classical biological control. -

Remarks on Some European Aleocharinae, with Description of a New Rhopaletes Species from Croatia (Coleoptera: Staphylinidae)
Travaux du Muséum National d’Histoire Naturelle © Décembre Vol. LIII pp. 191–215 «Grigore Antipa» 2010 DOI: 10.2478/v10191-010-0015-6 REMARKS ON SOME EUROPEAN ALEOCHARINAE, WITH DESCRIPTION OF A NEW RHOPALETES SPECIES FROM CROATIA (COLEOPTERA: STAPHYLINIDAE) LÁSZLÓ ÁDÁM Abstract. Based on an examination of type and non-type material, ten species-group names are synonymised: Atheta mediterranea G. Benick, 1941, Aloconota carpathica Jeannel et Jarrige, 1949 and Atheta carpatensis Tichomirova, 1973 with Aloconota mihoki (Bernhauer, 1913); Amischa jugorum Scheerpeltz, 1956 with Amischa analis (Gravenhorst, 1802); Amischa strupii Scheerpeltz, 1967 with Amischa bifoveolata (Mannerheim, 1830); Atheta tricholomatobia V. B. Semenov, 2002 with Atheta boehmei Linke, 1934; Atheta palatina G. Benick, 1974 and Atheta palatina G. Benick, 1975 with Atheta dilaticornis (Kraatz, 1856); Atheta degenerata G. Benick, 1974 and Atheta degenerata G. Benick, 1975 with Atheta testaceipes (Heer, 1839). A new name, Atheta velebitica nom. nov. is proposed for Atheta serotina Ádám, 2008, a junior primary homonym of Atheta serotina Blackwelder, 1944. A revised key for the Central European species of the Aloconota sulcifrons group is provided. Comments on the separation of the males of Amischa bifoveolata and A. analis are given. A key for the identification of Amischa species occurring in Hungary and its close surroundings is presented. Remarks are presented about the relationships of Alevonota Thomson, 1858 and Enalodroma Thomson, 1859. The taxonomic status of Oxypodera Bernhauer, 1915 and Mycetota Ádám, 1987 is discussed. The specific status of Pella hampei (Kraatz, 1862) is debated. Remarks are presented about the relationships of Alevonota Thomson, 1858, as well as Mycetota Ádám, 1987, Oxypodera Bernhauer, 1915 and Rhopaletes Cameron, 1939. -

First Record of the Zoophytophagous Plant Bug Atractotomus Mali (Hemiptera: Miridae) in Quebec Orchards
Document généré le 27 sept. 2021 04:22 Phytoprotection First record of the zoophytophagous plant bug Atractotomus mali (Hemiptera: Miridae) in Quebec orchards Première mention de la punaise zoophytophage Atractotomus mali (Hemiptera: Miridae) dans les vergers québécois Julien Saguez, Jacques Lasnier, Michael D. Schwartz et Charles Vincent Volume 95, numéro 1, 2015 Résumé de l'article Atractotomus mali (Meyer-Dür, 1843) (Hemiptera: Miridae) est un insecte URI : https://id.erudit.org/iderudit/1035303ar zoophytophage associé aux vergers en Europe et en Amérique du Nord. Au DOI : https://doi.org/10.7202/1035303ar Canada, il a été rapporté dans les vergers de pommiers (Malus domestica Borkh) de plusieurs provinces, mais principalement en Nouvelle-Écosse où il a Aller au sommaire du numéro induit plus de dommage sur les fruits que d’effets de prédation. Durant l’été 2014, nous avons récolté 33 spécimens dans un verger de Magog (Qc, Canada) en utilisant la méthode du battage. Cette étude constitue la première mention Éditeur(s) d’Atractotomus mali au Québec. Société de protection des plantes du Québec (SPPQ) ISSN 1710-1603 (numérique) Découvrir la revue Citer cet article Saguez, J., Lasnier, J., Schwartz, M. D. & Vincent, C. (2015). First record of the zoophytophagous plant bug Atractotomus mali (Hemiptera: Miridae) in Quebec orchards. Phytoprotection, 95(1), 38–40. https://doi.org/10.7202/1035303ar Tous droits réservés © La société de protection des plantes du Québec, 2015 Ce document est protégé par la loi sur le droit d’auteur. L’utilisation des services d’Érudit (y compris la reproduction) est assujettie à sa politique d’utilisation que vous pouvez consulter en ligne. -

Carabids and Other Beneficial Arthropods in Cereal Crops and Permanent Grasslands and Influence of Field and Landscape Parameters D
Carabids and other beneficial arthropods in cereal crops and permanent grasslands and influence of field and landscape parameters D. Massaloux To cite this version: D. Massaloux. Carabids and other beneficial arthropods in cereal crops and permanent grasslands and influence of field and landscape parameters. Biodiversity and Ecology. AgroParisTech, 2020. English. tel-02886480v2 HAL Id: tel-02886480 https://hal-agroparistech.archives-ouvertes.fr/tel-02886480v2 Submitted on 9 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2020 IAVF 0012 THESE DE DOCTORAT préparée à l’Institut des sciences et industries du vivant et de l’environnement (AgroParisTech) pour obtenir le grade de Docteur de l’Institut agronomique vétérinaire et forestier de France Spécialité : Écologie École doctorale n°581 Agriculture, alimentation, biologie, environnement et santé (ABIES) par Damien MASSALOUX Influence du paysage et de la parcelle sur les diversités de carabes et d’autres arthropodes en céréales et prairies permanentes Directeur de thèse : Alexander Wezel Co-encadrement de la thèse : Benoit Sarrazin Thèse présentée et soutenue à Lyon le 22 juin 2020 Composition du jury : M. Pierre-Henri Gouyon, Professeur, Muséum National d’Histoire Naturelle Rapporteur M. -

First Record of the Zoophytophagous Plant Bug Atractotomus Mali (Hemiptera: Miridae) in Quebec Orchards
Document generated on 09/27/2021 10:22 p.m. Phytoprotection First record of the zoophytophagous plant bug Atractotomus mali (Hemiptera: Miridae) in Quebec orchards Première mention de la punaise zoophytophage Atractotomus mali (Hemiptera: Miridae) dans les vergers québécois Julien Saguez, Jacques Lasnier, Michael D. Schwartz and Charles Vincent Volume 95, Number 1, 2015 Article abstract Atractotomus mali (Meyer-Dür, 1843) (Hemiptera: Miridae) is a URI: https://id.erudit.org/iderudit/1035303ar zoophytophagous insect associated with orchards in Europe and North DOI: https://doi.org/10.7202/1035303ar America. In Canada, it has previously been reported in apple (Malus domestica Borkh) orchards in several provinces, but mainly in Nova Scotia, where it See table of contents induced more damage on fruit than predatory effects. During the summer of 2014, we collected 33 specimens in an apple orchard in Magog (QC, Canada), using a tapping method. This study constitutes the first record of A. mali in Publisher(s) Quebec. Société de protection des plantes du Québec (SPPQ) ISSN 1710-1603 (digital) Explore this journal Cite this article Saguez, J., Lasnier, J., Schwartz, M. D. & Vincent, C. (2015). First record of the zoophytophagous plant bug Atractotomus mali (Hemiptera: Miridae) in Quebec orchards. Phytoprotection, 95(1), 38–40. https://doi.org/10.7202/1035303ar Tous droits réservés © La société de protection des plantes du Québec, 2015 This document is protected by copyright law. Use of the services of Érudit (including reproduction) is subject to its terms and conditions, which can be viewed online. https://apropos.erudit.org/en/users/policy-on-use/ This article is disseminated and preserved by Érudit. -

Its Breadth of Effectiveness Against Predators J
J. Appl. Entomol. Haemolymph defence of an invasive herbivore: its breadth of effectiveness against predators J. G. Lundgren1, S. Toepfer2,3, T. Haye2 & U. Kuhlmann2 1 USDA-ARS, North Central Agricultural Research Laboratory, Brookings, SD, USA 2 CABI Europe-Switzerland, Delemont, Switzerland 3 CABI Europe, c/o Plant Health Service, CABI Europe, Hodmezovasarhely, Hungary Keywords Abstract Tetramorium caespitum, Zea mays, biological control, Carabidae, diel cycle, Lycosidae Defensive characteristics of organisms affect the trophic linkages within food webs and influence the ability of invasive species to expand their Correspondence range. Diabrotica v. virgifera is one such invasive herbivore whose preda- Jonathan Lundgren (corresponding author), tor community is restricted by a larval haemolymph defence. The effec- NCARL, USDA-ARS, 2923 Medary Avenue, tiveness of this haemolymph defence against a range of predator Brookings, SD, USA. E-mail: functional and taxonomic guilds from the recipient biota was evaluated [email protected] in a series of experiments. Eight predator species (Carabidae, Lycosidae, Received: August 5, 2009; accepted: October Formicidae) were fed D. v. virgifera 3rd instars or equivalent-sized mag- 28, 2009. gots in the laboratory, and the mean times spent eating, cleaning their mouthparts, resting and walking following attacks on each prey were doi: 10.1111/j.1439-0418.2009.01478.x compared. Prey species were restrained in five Hungarian maize fields for 1 h periods beginning at 09:00 and 22:00 hours. The proportion of each species attacked and the number and identity of predators consum- ing each prey item were recorded. All predators spent less time eating D.