Positive and Negative Interactions Control a Facilitation Cascade 1,2, 3 � 1,2,4 3 3 PAUL E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dedication Donald Perrin De Sylva
Dedication The Proceedings of the First International Symposium on Mangroves as Fish Habitat are dedicated to the memory of University of Miami Professors Samuel C. Snedaker and Donald Perrin de Sylva. Samuel C. Snedaker Donald Perrin de Sylva (1938–2005) (1929–2004) Professor Samuel Curry Snedaker Our longtime collaborator and dear passed away on March 21, 2005 in friend, University of Miami Professor Yakima, Washington, after an eminent Donald P. de Sylva, passed away in career on the faculty of the University Brooksville, Florida on January 28, of Florida and the University of Miami. 2004. Over the course of his diverse A world authority on mangrove eco- and productive career, he worked systems, he authored numerous books closely with mangrove expert and and publications on topics as diverse colleague Professor Samuel Snedaker as tropical ecology, global climate on relationships between mangrove change, and wetlands and fish communities. Don pollutants made major scientific contributions in marine to this area of research close to home organisms in south and sedi- Florida ments. One and as far of his most afield as enduring Southeast contributions Asia. He to marine sci- was the ences was the world’s publication leading authority on one of the most in 1974 of ecologically important inhabitants of “The ecology coastal mangrove habitats—the great of mangroves” (coauthored with Ariel barracuda. His 1963 book Systematics Lugo), a paper that set the high stan- and Life History of the Great Barracuda dard by which contemporary mangrove continues to be an essential reference ecology continues to be measured. for those interested in the taxonomy, Sam’s studies laid the scientific bases biology, and ecology of this species. -

Redalyc.Feeding Ecology of the Planehead Filefish Stephanolepis
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Mancera-Rodríguez, Néstor Javier; Castro-Hernández, José Juan Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Revista de Biología Marina y Oceanografía, vol. 50, núm. 2, agosto, 2015, pp. 221-234 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47941332002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía Vol. 50, Nº2: 221-234, agosto 2015 DOI 10.4067/S0718-19572015000300002 ARTICLE Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Ecología trófica de Stephanolepis hispidus (Pisces: Monacanthidae), en el área de las Islas Canarias Néstor Javier Mancera-Rodríguez1 and José Juan Castro-Hernández2 1Universidad Nacional de Colombia, Department of Forestry Sciences, Calle 59A No. 63-20, Bloque 20, oficina 211, Medellín, Colombia. [email protected] 2Universidad de Las Palmas de Gran Canaria, Department of Biology, Edf. Ciencias Básicas, Campus de Tafira, 35017, Las Palmas de Gran Canaria, Islas Canarias, España. [email protected] Resumen.- Se examinó la ecología alimentaria de Stephanolepis hispidus, en aguas de las Islas Canarias. El estudio se basó en el contenido estomacal de 823 ejemplares de 8,9 cm a 25,9 cm de longitud total (LT), capturados mensualmente en trampas para peces entre febrero de 1998 y junio de 1999. -

5-Review-Fish-Habita
United Nations UNEP/GEF South China Sea Global Environment Environment Programme Project Facility UNEP/GEF/SCS/RWG-F.8/5 Date: 12th October 2006 Original: English Eighth Meeting of the Regional Working Group for the Fisheries Component of the UNEP/GEF Project: “Reversing Environmental Degradation Trends in the South China Sea and Gulf of Thailand” Bangka Belitung Province, Indonesia 1st - 4th November 2006 INFORMATION COLLATED BY THE FISHERIES AND HABITAT COMPONENTS OF THE SOUTH CHINA SEA PROJECT ON SITES IMPORTANT TO THE LIFE- CYCLES OF SIGNIFICANT FISH SPECIES UNEP/GEF/SCS/RWG-F.8/5 Page 1 IDENTIFICATION OF FISHERIES REFUGIA IN THE GULF OF THAILAND It was discussed at the Sixth Meeting of the Regional Scientific and Technical Committee (RSTC) in December 2006 that the Regional Working Group on Fisheries should take the following two-track approach to the identification of fisheries refugia: 1. Review known spawning areas for pelagic and invertebrate species, with the aim of evaluating these sites as candidate spawning refugia. 2. Evaluate each of the project’s habitat demonstration sites as potential juvenile/pre-recruit refugia for significant demersal species. Rationale for the Two-Track Approach to the Identification of Fisheries Refugia The two main life history events for fished species are reproduction and recruitment. It was noted by the RSTC that both of these events involve movement between areas, and some species, often pelagic fishes, migrate to particular spawning areas. It was also noted that many species also utilise specific coastal habitats such as coral reefs, seagrass, and mangroves as nursery areas. In terms of the effects of fishing, most populations of fished species are particularly vulnerable to the impacts of high levels of fishing effort in areas and at times where there are high abundances of (a) stock in spawning condition, (b) juveniles and pre-recruits, or (c) pre-recruits migrating to fishing grounds. -
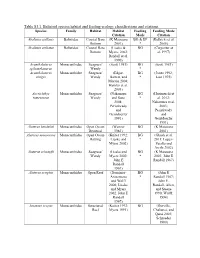
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

笛鯛科 Lutjanidae 紅鮪 Mangrove Red Snapper (Lutjanus Argentimaculatus ) 65 紅魚 Malabar Blood Snapper (Lutjanus Malabaricus) 67 石蚌 Star Snapper (Lutjanus Stellatus) 69
Identification街 市 Guide to圖 鑑 Live Reef Food Fish 活珊瑚魚 in Hong Kong’s Wet MarketS 1 2 出版機構: Published by: 合作伙伴: In partnership with: 關於作者: About the authors 侯卓儒 是香港大學的研究碩士生,研究項目 Loby C. Y. Hau is an M.Phil candidate at The 為蘇眉魚 (Cheilinus undulatus) 在香港的貿易 University of Hong Kong, with a research focus on 情況。 the Humphead wrasse (Cheilinus undulatus) trade in Hong Kong. 何嘉欣 是 BLOOM Association Hong Kong 的海洋項目經理。 Kathleen K. Y. Ho is the Marine Programme Manager at BLOOM Association Hong Kong. 佘國豪 是 BLOOM Association Hong Kong 的海洋項目總監,研究珊瑚魚及其貿易情況 Stan K. H. Shea is the Marine Programme 已有逾十年的經驗。 Director at BLOOM Association Hong Kong, with more than 10 years’ research experience in reef fish and their trade. 請瀏覽以下網址了解如何在香港「識揀海鮮」: For more information on sustainable seafood in Hong Kong, please visit: www.chooserighttoday.org 3 目錄 Contents 致謝 Acknowledgements 1 引言 Introduction 3 如何使用此圖鑑 How To Use This Guide 5 魚的形態 Fish morphology 12 魚身的顏色花紋 Fish color patterns 13 尾鰭的形狀 Caudal fin shapes 14 魚的體形 Fish body shapes 15 鰺科 Carangidae 黃鶘䱽 Snubnose pompano (Trachinotus blochii) 17 石斑科 Epinephelidae 沙巴龍躉 Sabah grouper (Epinephelus lanceolatus x Epinephelus fuscoguttatus) 19 黑瓜子 Redmouth grouper (Aethaloperca rogaa) 21 烏絲斑 Chocolate hind (Cephalopholis boenak) 23 蕃茄斑 Tomato hind (Cephalopholis sonnerati) 25 老鼠斑 Humpback grouper (Cromileptes altivelis) 27 紅斑 Hong Kong grouper (Epinephelus akaara) 29 黃釘 Yellow grouper (Epinephelus awoara) 31 芝麻斑 Duskytail grouper (Epinephelus bleekeri) 33 青斑 Orange-spotted grouper (Epinephelus coioides) 35 蘇鼠斑 Coral -

Seagrass Herbivory: Evidence for the Continued Grazing of Marine Grasses
MARINE ECOLOGY PROGRESS SERIES Vol. 176: 291-302, 1999 Published January 18 Mar Ecol Prog Ser REVIEW Seagrass herbivory: evidence for the continued grazing of marine grasses John F. Valentine*, K. L. Heck, Jr 101 Bienville Boulevard, Dauphin Island Sea Lab, Dauphin Island, Alabama 36528-0369, USA and Department of Marine Science, University of South Alabama, Mobile, Alabama 36688-0002, USA ABSTRACT: Unlike the majority of marine plants, seagrasses are believed to experience little damage from the feeding activities of marine herbivores. Based on our previous work, plus a review of the lit- erature, we suggest that this paradigm significantly underestimates the importance of seagrass her- bivory in nearshore environments. In this review, we provide evidence from over 100 publications, showing that grazing on seagrasses is widespread in the world's oceans. Overwhelmingly, reports of grazing on seagrasses are based on observations, laboratory measurements, and bioenergetic calcula- tions. To date, few field experiments have been conducted to evaluate the importance of seagrass graz- ing in the nearshore environment. Of these, even fewer have considered the possibility that herbivores may stimulate rates of primary production of the role of belowground nutrient reserves in determining the impacts of grazers on seagrasses. We contend that the currently accepted view that herbivory plays a minor role in the energetics of seagrass habitats needs to be reexamined by measuring seagrass responses to grazer induced tissue losses in controlled field manipulations. Only then will we be able to determine the in~portanceof the seagrass-grazing pathway in marine food webs. KEY WORDS: Seagrass . Herbivory . Waterfowl . Fishes . -

Monacanthus Chinensis (Osbeck, 1765) En - Fanbellied Leatherjacket
click for previous page 3940 Bony Fishes Colurodontis paxmani Hutchins, 1977 En - Paxman’s leatherjacket. Maximum total length about 15 cm. Inhabits shallow seagrass beds. Found in northern Western Australia to Cape York. (after Hutchins, 1977) Eubalichthys mosaicus (Ramsay and Ogilby, 1886) En - Mosaic leatherjacket. Maximum total length about 60 cm. Inhabits inshore and offshore reefs to depths of 170 m. Marketed occasionally in southeastern Australia. Found in Australia’s southern half. (after Allen, 1985) Monacanthus chinensis (Osbeck, 1765) En - Fanbellied leatherjacket. Maximum total length about 38 cm. Inhabits shallow seagrass beds and coastal reefs. Marketed in Australia, Indonesia, and the Philippines. Occurs in northern Australia, New Guinea, Indonesia, Malayasia, Thailand, Philippines, China (including Taiwan Province), and southern Japan. (after Schroeder, 1980) Tetraodontiformes: Monacanthidae 3941 Nelusetta ayraudi (Quoy and Gaimard, 1824) En - Chinaman leatherjacket. Maximum total length about 71 cm. Inhabits shallow embayments as a juvenile, adult moving into offshore waters at depths to 120 m. Marketed in southeastern Australia. Found only in southern Australia. (after Kailola et al., 1990) Oxymonacanthus longirostris (Bloch and Scheider, 1801) En - Harlequin filefish. Maximum total length about 9 cm. Inhabits shallow coral reefs. A popular aquarium fish. Widespread in the Indo-West Pacific, from East Africa to Samoa, and northern Australia to southern Japan. (after Schroeder, 1980) Paraluteres prionurus (Bleeker, 1851) En - Blacksaddle filefish. Maximum total length about 10 cm. Inhabits shallow coral reefs. Widespread throughout the Indo-West Pacific, from East Africa to the Marshall Islands, and from northern Australia to southern Japan. (after Debelius, 1993) 3942 Bony Fishes P a ra m o na c an t hu (Bs leeck erho, 185 i 2)ro ce p ha l us En - Pigface leatherjacket. -

Chec List an Update to the List of Coral Reef Fishes from Koh Tao, Gulf Of
Check List 10(5): 1123–1133, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution An update to the list PECIES S Gulf of Thailand OF of coral reef fishes from Koh Tao, 1* 2 ISTS Patrick Scaps and Chad Scott L 1 Laboratoire de Biologie animale,[email protected] Université des Sciences et Technologies de Lille, 59 655 Villeneuve d’Ascq Cédex, France. 2 New Heaven Reef Conservation Program, 48 Moo 3, Koh Tao, Suratthani, Thailand, 84360. * Corresponding author: E-mail: ABSTRACT: (i.e., cryptic species or transient Twenty-one species are reported for the first time from Koh Tao (Turtle Island) in the Gulf of Thailand. TInformationhis and photographs were obtained from local scuba divers in order to censusAntennatus rare and Histrio), Ophichthyidae (genusspecies onlyCallechelys present), duringPlatycephalidae one season) (genus or not previouslyThysanophrys recorded), Plotosidae fish species (genus living Plotosus on or near) and coral Synanceiidae reefs from the(genera area. Inimicus is the and first Synanceia time that species belonging to the families AntennariidaePseudobalistes, (genera Balistidae; Cyclichthys, Diodontidae; Bolbometopon, Scaridae; and Hippocampus, ), and reef-fish genera of severalAntennatus families nummifer ( (Antennariidae), Pseudobalistes marginatus (Balistidae), Monacanthus chinensis (Monacanthidae),Syngnathidae), Callechelys among others,marmora haveta been(Ophichthyidae), recorded in KohThysnophrys Tao. Of the cf. 21 chiltonae species reported(Platycephalidae), for the first Bolbometopon time from muricatumKoh Tao, 7 (Scaridae) species ( and Synanceia cf. verrucosa (Synanceidae)) are new records for the species found in the present study. Gulf of Thailand. To date, 223 species of coral reef fishes belonging to 53 families are known from Koh Tao, including the 10.15560/10.5.1123 DOI: Introduction MaterialS and Methods 2 archipelago in the western Gulf of Thailand. -

2020 Special Issue
Journal Home page : www.jeb.co.in « E-mail : [email protected] Original Research Journal of Environmental Biology TM p-ISSN: 0254-8704 e-ISSN: 2394-0379 JEB CODEN: JEBIDP DOI : http://doi.org/10.22438/jeb/41/5(SI)/MS_23 White Smoke Plagiarism Detector Just write. DNA barcoding analysis of larval fishes in Peninsular Malaysia I.A. Azmir1, Y.B. Esa2,3*, S.M.N. Amin2,4, M.Y.I. Salwany2 and M.Y.F. Zuraina5 1School of Biology, Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM) Negeri Sembilan, Kampus Kuala Pilah, Pekan Parit Tinggi, 72000 Kuala Pilah, Negeri Sembilan, Malaysia 2Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor Malaysia 3International Institute of Aquaculture and Aquatic Sciences (I-AQUAS), Universiti Putra Malaysia, 71050 port Dickson, Negeri Sembilan, Malaysia 4FAO World Fisheries University, Pukyong National University, Busan, 48547, South Korea 5Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM), Shah Alam, Selangor, Malaysia *Corresponding Author Email : [email protected] Paper received: 15.12.2020 Revised received: 17.03.2020 Accepted: 14.08.2020 Abstract Aim: To identify fish larvae to species level by DNA barcoding method using the mitochondrial Cytochrome c Oxidase I (COI) gene. The lack of species identification work on fish larvae in Peninsular Malaysia has warrant this study to be conducted. Methodology: Identification of fish larvae species from mangrove areas of Pendas Johor, Matang Perak, Pekan Pahang and Setiu Terengganu was conducted. Samples were collected from April 2015 to September 2015 using a bongo net, towed at a depth of about 0.5 m from the surface for 5 min against the tidal flow. -

Canning Estuary
DDeevveellooppmmeenntt ooff bbiioottiicc iinnddiicceess ffoorr eessttaabblliisshhiinngg aanndd mmoonniittoorriinngg eeccoossyysstteemm hheeaalltthh ooff tthhee SSwwaann-- CCaannnniinngg EEssttuuaarryy FINAL TECHNICAL REPORT May 2011 Valesini, F.J., Hallett, C.S., Cottingham, A., Hesp, S.A., Hoeksema, S.D., Hall, N.G., Linke, T.E., Buckland, A.J. Prepared for the Swan River Trust, Department of Water and Department of Fisheries 1 2 Development of biotic indices for establishing and monitoring ecosystem health of the Swan- Canning Estuary FINAL TECHNICAL REPORT May 2011 Valesini, F.J., Hallett, C.S., Cottingham, A., Hesp, S.A., Hoeksema, S.D., Hall, N.G., Linke, T.E., Buckland, A.J. Prepared for the Swan River Trust, Department of Water and Department of Fisheries 3 4 Disclaimer The authors have prepared this report in accordance with the usual care and thoroughness of the Centre for Fish and Fisheries Research (CFFR) for the use by the Swan River Trust (SRT), Department of Water (DoW) and Department of Fisheries (DoF) and only those third parties who have been authorised in writing by the CFFR to rely on the report. It is based on generally accepted practices and standards at the time it was prepared. No other warranty, expressed or implied, is made as to the professional advice included in this report. It is prepared in accordance with the scope of work and for the purpose required by the SRT, DoW and DoF. The methodology adopted and sources of information used by the authors are outlined in this report. The authors have made no independent verification of this information beyond the agreed scope of works, and they assume no responsibility for any inaccuracies or omissions. -

View/Download
TETRAODONTIFORMES (part 2) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 1.0 - 30 Nov. 2020 Order TETRAODONTIFORMES (part 2 of 2) Suborder MOLOIDEI Family MOLIDAE Molas or Ocean Sunfishes 3 genera · 5 species Masturus Gill 1884 mast-, mastoid; oura, tail, referring to caudal fin (clavus) “extended backwards at the subaxial or submedian rays, and assuming a mastoid shape” Masturus lanceolatus (Liénard 1840) lanceolate, referring to shape of clavus (where dorsal and anal fins merge), forming a tail-like triangular lobe Mola Koelreuter 1766 millstone, referring to its somewhat circular shape (not tautonymous with Tetraodon mola Linnaeus 1758 since Koelreuter proposed a new species, M. aculeatus, actually a juvenile M. mola) Mola alexandrini (Ranzani 1839) in honor of Antonio Alessandrini (1786-1861, note latinization of name), Italian physician and anatomist, author of a detailed anatomical study of Mola gills published later that year [previously known as M. ramsayi] Mola mola (Linnaeus 1758) millstone, referring to its somewhat circular shape Mola tecta Nyegaard, Sawai, Gemmell, Gillum, Loneragan, Yamanoue & Stewart 2017 disguised or hidden, referring to how this species “evaded discovery for nearly three centuries, despite the keen interest among early sunfish taxonomists and the continued attention these curious fish receive” Ranzania Nardo 1840 -ia, belonging to: Camillo Ranzani (1775-1841), priest, naturalist and director of the Museum of Natural History of Bologna, for being the first to recognize Molidae as a distinct family [although authorship of family dates to Bonaparte 1835], and for “many other titles of merit in various branches of zoology” (translation) Ranzania laevis (Pennant 1776) smooth, referring to smooth skin covered with small, hard, hexagonal plates Mola alexandrini. -

New Records of Fish Parasitic Isopods (Crustacea: Isopoda) from the Gulf of Thailand
animals Article New Records of Fish Parasitic Isopods (Crustacea: Isopoda) from the Gulf of Thailand Watchariya Purivirojkul * and Apiruedee Songsuk Animal Systematics and Ecology Speciality Research Unit, Department of Zoology, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand; [email protected] * Correspondence: [email protected] Received: 5 November 2020; Accepted: 2 December 2020; Published: 4 December 2020 Simple Summary: Parasitic isopods were reported found from marine fishes from many habitat in the world. In Thailand, there is not much study on this parasitic group. This work has compiled all published parasitic isopods documents in Thailand from year 1950 to present include collecting samples from the Gulf of Thailand during the period 2006–2019. New host records were found from four species of parasitic isopods (Cymothoa eremita, Smenispa irregularis, Nerocila sundaica, Norileca triangulata) and two species of parasitic isopods (Argathona macronema, Norileca triangulata) were found first time in the central Indo-Pacific region. Abstract: From a total of 4140 marine fishes examined, eight species of parasitic isopods were reported from marine fishes in the Gulf of Thailand. These isopods were identified in two families, Corallanidae (Argathona macronema and Argathona rhinoceros) and Cymothoidae (Cymothoa eremita, Cymothoa elegans, Smenispa irregularis, Nerocila sundaica, Norileca indica and Norileca triangulata). Most of these parasitic isopods were found in the buccal cavity of their fish hosts with one host recorded as follows: C. eremita was found from Nemipterus hexodon, C. elegans was found from Scatophagus argus, N. sundaica was found from Saurida tumbil. The majority of the isopod specimens recorded in this study was S.