BONNER ZOOLOGISCHE MONOGRAPHIEN, Nr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
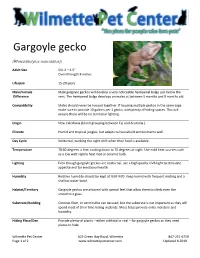
Gargoyle Gecko
Gargoyle gecko (Rhacodactylus auriculatus)) Adult Size SVL 4 – 4.5” Overall length 8 inches Lifespan 15-20 years Male/Female Male gargoyle geckos will develop a very noticeable hemipenal bulge just below the Difference vent. The hemipenal bulge develops on males at between 5 months and 9 months old. Compatibility Males should never be housed together. If housing multiple geckos in the same cage make sure to provide 10 gallons per 1 gecko, with plenty of hiding spaces. This will ensure there will be no territorial fighting. Origin New Caledonia (Island grouping between Fiji and Australia.) Climate Humid and tropical jungles, but adapts to household environments well. Day Cycle Nocturnal, working the night shift when their food is available. Temperature 78-82 degrees is fine, cooling down to 70 degrees at night. Use mild heat sources such as a low watt reptile heat mat or ceramic bulb. Lighting Even though gargoyle geckos are nocturnal, use a high quality UVA light to stimulate appetite and for emotional health. Humidity Relative humidity should be kept at %50-%70. Keep humid with frequent misting and a shallow water bowl. Habitat/Territory Gargoyle geckos are arboreal with special feet that allow them to climb even the smoothest glass. Substrate/Bedding Coconut fiber, or vermiculite can be used, but the substrate is not important as they will spend most of their time hiding in plants. Moss helps provide extra moisture and humidity. Hiding Place/Den Provide plenty of plants – either artificial or real – for gargoyle geckos as they need places to hide. Wilmette Pet Center 625 Green Bay Road, Wilmette 847-251-6750 Page 1 of 2 www.wilmettepetcenter.com Updated 4.2019 Cage Type Ten gallon aquariums or critter cages with screen tops work well for gargoyle geckos. -

THE UNDERCROFT We Must Expire in Hope{ of Resurrection to Life Again
THE UNDERCROFT We must Expire in hope{ of Resurrection to Life Again 1 Editorial Illu{tration{ Daniel Sell Matthew Adams - 43 Jeremy Duncan - 51, 63 Anxious P. - 16 2 Skinned Moon Daughter Cedric Plante - Cover, 3, 6, 8, 9 Benjamin Baugh Sean Poppe - 28 10 101 Uses of a Hanged Man Barry Blatt Layout Editing & De{ign Daniel Sell 15 The Doctor Patrick Stuart 23 Everyone is an Adventurer E{teemed Con{ultant{ Daniel Sell James Maliszewski 25 The Sickness Luke Gearing 29 Dead Inside Edward Lockhart 41 Cockdicktastrophe Chris Lawson 47 Nine Summits and the Matter of Birth Ezra Claverie M de in elson Ma ia S t y a it mp ic of Authent Editorial Authors live! Hitler writes a children’s book, scratches his name off and hides it among the others. Now children grow up blue and blond, unable to control their minds, the hidden taint in friendly balloons and curious caterpillars wormed itself inside and reprogrammed them into literary sleeper agents ready to steal our freedom. The swine! We never saw it coming. If only authors were dead. But if authors were dead you wouldn’t be able to find them, to sit at their side and learn all they had to say on art and beautiful things, on who is wrong and who is right, on what is allowed and what is ugly and what should be done about this and that. We would have only words and pictures on a page, if we can’t fill in the periphery with context then how will we understand anything? If they don’t tell us what to enjoy, how to correctly enjoy it, then how will we know? Who is right and who is mad? Whose ideas are poisonous and wrong? Call to arms! Stop that! Help me! We must slather ourselves in their selfish juices. -

Hollow-Bearing Trees As a Habitat Resource Along an Urbanisation Gradient
Hollow-Bearing Trees as a Habitat Resource along an Urbanisation Gradient Author Treby, Donna Louise Published 2014 Thesis Type Thesis (PhD Doctorate) School Griffith School of Environment DOI https://doi.org/10.25904/1912/1674 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/367782 Griffith Research Online https://research-repository.griffith.edu.au Hollow-bearing Trees as a Habitat Resource along an Urbanisation Gradient Donna Louise Treby MPhil (The University of Queensland) Environmental Futures Centre. Griffith School of Environment, Griffith University, Gold Coast. A thesis submitted for the fulfilment for the requirements of the degree of Doctor of Philosophy. December 2013. “If we all did the things we are capable of doing. We would literally live outstanding lives. I think; if we all lived our lives this way, we would truly create an amazing world.” Thomas Edison. i Acknowledgements: It would be remiss of me if I did not begin by acknowledging my principal supervisor Dr Guy Castley, for the inception, development and assistance with the completion of this study. Your generosity, open door policy and smiling face made it a pleasure to work with you. I owe you so much, but all I can give you is my respect and heartfelt thanks. Along with my associate supervisor Prof. Jean-Marc Hero their joint efforts inspired me and opened my mind to the complexities and vagaries of ecological systems and processes on such a large scale. To my volunteers in the field, Katie Robertson who gave so much of her time and help in the early stages of my project, Agustina Barros, Ivan Gregorian, Sally Healy, Guy Castley, Katrin Lowe, Kieran Treby, Phil Treby, Erin Wallace, Nicole Glenane, Nick Clark, Mark Ballantyne, Chris Tuohy, Ryan Pearson and Nickolas Rakatopare all contributed to the collection of data for this study. -

Keystone Species: the Concept and Its Relevance for Conservation Management in New Zealand
Keystone species: the concept and its relevance for conservation management in New Zealand SCIENCE FOR CONSERVATION 203 Ian J. Payton, Michael Fenner, William G. Lee Published by Department of Conservation P.O. Box 10-420 Wellington, New Zealand Science for Conservation is a scientific monograph series presenting research funded by New Zealand Department of Conservation (DOC). Manuscripts are internally and externally peer-reviewed; resulting publications are considered part of the formal international scientific literature. Titles are listed in the DOC Science Publishing catalogue on the departmental website http:// www.doc.govt.nz and printed copies can be purchased from [email protected] © Copyright July 2002, New Zealand Department of Conservation ISSN 11732946 ISBN 047822284X This report was prepared for publication by DOC Science Publishing, Science & Research Unit; editing by Lynette Clelland and layout by Ruth Munro. Publication was approved by the Manager, Science & Research Unit, Science Technology and Information Services, Department of Conservation, Wellington. CONTENTS Abstract 5 1. Introduction 6 2. Keystone concepts 6 3. Types of keystone species 8 3.1 Organisms controlling potential dominants 8 3.2 Resource providers 10 3.3 Mutualists 11 3.4 Ecosystem engineers 12 4. The New Zealand context 14 4.1 Organisms controlling potential dominants 14 4.2 Resource providers 16 4.3 Mutualists 18 4.4 Ecosystem engineers 19 5. Identifying keystone species 20 6. Implications for conservation management 21 7. Acknowledgements 22 8. References 23 4 Payton et al.Keystone species: the concept and its relevance in New Zealand Keystone species: the concept and its relevance for conservation management in New Zealand Ian J. -

Nature Conservation (Wildlife) Regulation 2006
Queensland Nature Conservation Act 1992 Nature Conservation (Wildlife) Regulation 2006 Current as at 1 September 2017 Queensland Nature Conservation (Wildlife) Regulation 2006 Contents Page Part 1 Preliminary 1 Short title . 5 2 Commencement . 5 3 Purpose . 5 4 Definitions . 6 5 Scientific names . 6 Part 2 Classes of native wildlife and declared management intent for the wildlife Division 1 Extinct in the wild wildlife 6 Native wildlife that is extinct in the wild wildlife . 7 7 Declared management intent for extinct in the wild wildlife . 8 8 Significance of extinct in the wild wildlife to nature and its value 8 9 Proposed management intent for extinct in the wild wildlife . 8 10 Principles for the taking, keeping or use of extinct in the wild wildlife 9 Division 2 Endangered wildlife 11 Native wildlife that is endangered wildlife . 10 12 Declared management intent for endangered wildlife . 10 13 Significance of endangered wildlife to nature and its value . 10 14 Proposed management intent for endangered wildlife . 11 15 Principles for the taking, keeping or use of endangered wildlife . 12 Division 3 Vulnerable wildlife 16 Native wildlife that is vulnerable wildlife . 13 17 Declared management intent for vulnerable wildlife . 13 18 Significance of vulnerable wildlife to nature and its value . 13 19 Proposed management intent for vulnerable wildlife . 14 20 Principles for the taking, keeping or use of vulnerable wildlife . 15 Nature Conservation (Wildlife) Regulation 2006 Contents Division 4 Near threatened wildlife 26 Native wildlife that is near threatened wildlife . 16 27 Declared management intent for near threatened wildlife . 16 28 Significance of near threatened wildlife to nature and its value . -

An Intial Estimation of the Numbers and Identification of Extant Non
Answers Research Journal 8 (2015):171–186. www.answersingenesis.org/arj/v8/lizard-kinds-order-squamata.pdf $Q,QLWLDO(VWLPDWLRQRIWKH1XPEHUVDQG,GHQWLÀFDWLRQRI Extant Non-Snake/Non-Amphisbaenian Lizard Kinds: Order Squamata Tom Hennigan, Truett-McConnell College, Cleveland, Georgia. $EVWUDFW %LRV\VWHPDWLFVLVLQJUHDWÁX[WRGD\EHFDXVHRIWKHSOHWKRUDRIJHQHWLFUHVHDUFKZKLFKFRQWLQXDOO\ UHGHÀQHVKRZZHSHUFHLYHUHODWLRQVKLSVEHWZHHQRUJDQLVPV'HVSLWHWKHODUJHDPRXQWRIGDWDEHLQJ SXEOLVKHGWKHFKDOOHQJHLVKDYLQJHQRXJKNQRZOHGJHDERXWJHQHWLFVWRGUDZFRQFOXVLRQVUHJDUGLQJ WKHELRORJLFDOKLVWRU\RIRUJDQLVPVDQGWKHLUWD[RQRP\&RQVHTXHQWO\WKHELRV\VWHPDWLFVIRUPRVWWD[D LVLQJUHDWIOX[DQGQRWZLWKRXWFRQWURYHUV\E\SUDFWLWLRQHUVLQWKHILHOG7KHUHIRUHWKLVSUHOLPLQDU\SDSHU LVmeant to produce a current summary of lizard systematics, as it is understood today. It is meant to lay a JURXQGZRUNIRUFUHDWLRQV\VWHPDWLFVZLWKWKHJRDORIHVWLPDWLQJWKHQXPEHURIEDUDPLQVEURXJKWRQ WKH $UN %DVHG RQ WKH DQDO\VHV RI FXUUHQW PROHFXODU GDWD WD[RQRP\ K\EULGL]DWLRQ FDSDELOLW\ DQG VWDWLVWLFDO EDUDPLQRORJ\ RI H[WDQW RUJDQLVPV D WHQWDWLYH HVWLPDWH RI H[WDQW QRQVQDNH QRQ DPSKLVEDHQLDQOL]DUGNLQGVZHUHWDNHQRQERDUGWKH$UN,WLVKRSHGWKDWWKLVSDSHUZLOOHQFRXUDJH IXWXUHUHVHDUFKLQWRFUHDWLRQLVWELRV\VWHPDWLFV Keywords: $UN(QFRXQWHUELRV\VWHPDWLFVWD[RQRP\UHSWLOHVVTXDPDWDNLQGEDUDPLQRORJ\OL]DUG ,QWURGXFWLRQ today may change tomorrow, depending on the data Creation research is guided by God’s Word, which and assumptions about that data. For example, LVIRXQGDWLRQDOWRWKHVFLHQWLÀFPRGHOVWKDWDUHEXLOW naturalists assume randomness and universal 7KHELEOLFDODQGVFLHQWLÀFFKDOOHQJHLVWRLQYHVWLJDWH -

RVC Exotics Service CRESTED GECKO CARE
RVC Exotics Service Royal Veterinary College Royal College Street London NW1 0TU T: 0207 554 3528 F: 0207 388 8124 www.rvc.ac.uk/BSAH CRESTED GECKO CARE The Crested gecko (Rhacodactylus ciliatus) originates from the islands of New Caledonia where the species was believed to be extinct until 1994 when it was subsequently rediscovered. In its natural environment, it can be found resting in rainforests, sleeping in trunk hollows or leaf litter, and only becomes active at night. Similar to other geckos, it can shed its tail as a defence mechanism (autotomy), but unlike others it lacks an ability to regenerate the tail, so care should be taken while handling. Geckos may live 10-15 years if looked after correctly. HOUSING • As large a vivarium as possible should be provided to enable room for exercise, and a thermal gradient to be created along the length of the tank (hot to cold). Wooden or fibreglass vivaria are ideal as this provides the lizard with some visual security and ventilation can be provided at lizard level. • Good ventilation is required and additional ventilation holes may need to be created. • Hides are required to provide some security. Artificial plants, cardboard boxes, plant pots, logs or commercially available hides can be used. They should be placed both at the warm and cooler ends of the tank. One hide should contain damp moss, kitchen towel or vermiculite to provide a humid environment for shedding. • Substrates suitable for housing lizards include newspaper, Astroturf and some of the commercially available substrates. It is important that the substrates either cannot be eaten, or if they are, do not cause blockages as this can prove fatal. -

A New Locality for Correlophus Ciliatus and Rhacodactylus Leachianus (Sauria: Diplodactylidae) from Néhoué River, Northern New Caledonia
Herpetology Notes, volume 8: 553-555 (2015) (published online on 06 December 2015) A new locality for Correlophus ciliatus and Rhacodactylus leachianus (Sauria: Diplodactylidae) from Néhoué River, northern New Caledonia Mickaël Sanchez1, Jean-Jérôme Cassan2 and Thomas Duval3,* Giant geckos from New Caledonia (Pacific Ocean) We observed seven native gecko species: Bavayia are charismatic nocturnal lizards. This paraphyletic (aff.) cyclura (n=1), Bavayia (aff.) exsuccida (n=1), group is represented by three genera, Rhacodactylus, Correlophus ciliatus (n=1), Dierrogekko nehoueensis Correlophus and Mniarogekko, all endemic to Bauer, Jackman, Sadlier and Whitaker, 2006 (n=1), New Caledonia (Bauer et al., 2012). Rhacodactylus Eurydactylodes agricolae Henkel and Böhme, 2001 leachianus (Cuvier, 1829) is largely distributed on the (n=1), Mniarogekko jalu Bauer, Whitaker, Sadlier and Grande Terre including the Île des Pins and its satellite Jackman, 2012 (n=1) and Rhacodactylus leachianus islands, whereas Correlophus ciliatus Guichenot, 1866 (n=1). Also, the alien Hemidactylus frenatus Dumeril is mostly known in the southern part of the Grande and Bibron, 1836 (n=3) has been sighted. The occurrence Terre, the Île des Pins and its satellite islands (Bauer of C. ciliatus and R. leachianus (Fig. 2 and 3) represent et al., 2012). Here, we report a new locality for both new records for this site. Both gecko species were species in the north-western part of Grande Terre, along observed close to the ground, at a height of less than the Néhoué River (Fig. 1). 1.5 m. The Néhoué River is characterized by gallery forests It is the first time that R. leachianus is recorded in the growing on deep alluvial soils. -

Dieter Thomas Tietze Editor How They Arise, Modify and Vanish
Fascinating Life Sciences Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Fascinating Life Sciences This interdisciplinary series brings together the most essential and captivating topics in the life sciences. They range from the plant sciences to zoology, from the microbiome to macrobiome, and from basic biology to biotechnology. The series not only highlights fascinating research; it also discusses major challenges associated with the life sciences and related disciplines and outlines future research directions. Individual volumes provide in-depth information, are richly illustrated with photographs, illustrations, and maps, and feature suggestions for further reading or glossaries where appropriate. Interested researchers in all areas of the life sciences, as well as biology enthusiasts, will find the series’ interdisciplinary focus and highly readable volumes especially appealing. More information about this series at http://www.springer.com/series/15408 Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Editor Dieter Thomas Tietze Natural History Museum Basel Basel, Switzerland ISSN 2509-6745 ISSN 2509-6753 (electronic) Fascinating Life Sciences ISBN 978-3-319-91688-0 ISBN 978-3-319-91689-7 (eBook) https://doi.org/10.1007/978-3-319-91689-7 Library of Congress Control Number: 2018948152 © The Editor(s) (if applicable) and The Author(s) 2018. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. -

Species Names in Phylogenetic Nomenclature
Syst. Biol. 48(4):790–807, 1999 Species Names in Phylogenetic Nomenclature PHILIP D. CANTINO,1* HAROLD N. BRYANT,2 KEVIN DE QUEIROZ,3 MICHAEL J. DONOGHUE,4 TORSTEN ERIKSSON,5 DAVID M. HILLIS,6 AND MICHAEL S. Y. LEE7 1Department of Environmental and Plant Biology, Ohio University, Athens, Ohio 45701, USA; E-mail: [email protected] 2Royal Saskatchewan Museum, 2340 Albert Street, Regina, Saskatchewan S4P 3V7, Canada; E-mail: [email protected] 3Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560, USA; E-mail: [email protected] 4Harvard University Herbaria, 22 Divinity Ave., Cambridge, Massachusetts 02138, USA; E-mail: [email protected] 5Bergius Foundation, Royal Swedish Academy of Sciences, Box 50017, 104 05 Stockholm, Sweden; E-mail: [email protected] 6Section of Integrative Biology, School of Biological Sciences, University of Texas, Austin, Texas 78712, USA; E-mail: [email protected] 7Department of Zoology, University of Queensland, Brisbane, Queensland 4072, Australia; E-mail: [email protected] Abstract.—Linnaean binomial nomenclature is logically incompatible with the phylogenetic nomenclature of de Queiroz and Gauthier (1992, Annu. Rev. Ecol. Syst. 23:449–480): The former is based on the concept of genus, thus making this rank mandatory, while the latter is based on phylo- genetic definitions and requires the abandonment of mandatory ranks. Thus, if species are to re- ceive names under phylogenetic nomenclature, a different method must be devised to name them. Here, 13 methods for naming species in the context of phylogenetic nomenclature are contrasted with each other and with Linnaean binomials. -

Microhabitat Preferences and Associated Behavior Patterns Of
Journal of Entomology and Zoology Studies 2019; 7(4): 924-928 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Microhabitat preferences and associated behavior JEZS 2019; 7(4): 924-928 © 2019 JEZS patterns of endemic pigmy lizard: Cophotis Received: 13-05-2019 Accepted: 16-06-2019 ceylanica in Horton plains, Sri Lanka WLR Keerthirathna Department of Zoology, University of Sri WLR Keerthirathna and WAD Mahaulpatha Jayewardenepura, Sri Lanka Abstract WAD Mahaulpatha The pigmy lizard (Cophotis ceylanica) is an endangered and rare lizard species endemic to Sri Lanka, yet Department of Zoology, University of Sri no studies exist on its microhabitat preferences. Therefore, the present study was carried out in the Cloud Jayewardenepura, Sri Lanka Forests of the Horton Plains National Park (HPNP) with the aim of addressing this knowledge gap in their ecology. Microhabitat variables were measured placing 1x1 m quadrates marking the point of each lizard sighting as the center and microhabitat details including perch plant characteristics, soil characteristics and environmental parameters were recorded. Highest number of individuals were seen on Sarcococca brevifolia (1.167±0.937) plant species. Total of 78.13%, C. ceylanica were observed perching on branches rather than on trunks or leaves. Highest percentage of pigmy lizards (48.87%) was recorded in the branches where the moss cover was between 50% - 75% and the lowest of 12.50% was recorded where the moss cover was less than 25%. Highest percentage of 71.88% of C. ceylanica were recorded perching in the height category of 2-3 m of perching plants. No individuals were recorded up to 1m from ground level. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica.