Thermal Ecology and Habitat Utilization of Rhacodactylus Leachianus from New Caledonia (Squamata: Diplodactylidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
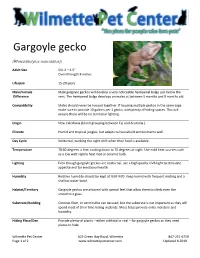
Gargoyle Gecko
Gargoyle gecko (Rhacodactylus auriculatus)) Adult Size SVL 4 – 4.5” Overall length 8 inches Lifespan 15-20 years Male/Female Male gargoyle geckos will develop a very noticeable hemipenal bulge just below the Difference vent. The hemipenal bulge develops on males at between 5 months and 9 months old. Compatibility Males should never be housed together. If housing multiple geckos in the same cage make sure to provide 10 gallons per 1 gecko, with plenty of hiding spaces. This will ensure there will be no territorial fighting. Origin New Caledonia (Island grouping between Fiji and Australia.) Climate Humid and tropical jungles, but adapts to household environments well. Day Cycle Nocturnal, working the night shift when their food is available. Temperature 78-82 degrees is fine, cooling down to 70 degrees at night. Use mild heat sources such as a low watt reptile heat mat or ceramic bulb. Lighting Even though gargoyle geckos are nocturnal, use a high quality UVA light to stimulate appetite and for emotional health. Humidity Relative humidity should be kept at %50-%70. Keep humid with frequent misting and a shallow water bowl. Habitat/Territory Gargoyle geckos are arboreal with special feet that allow them to climb even the smoothest glass. Substrate/Bedding Coconut fiber, or vermiculite can be used, but the substrate is not important as they will spend most of their time hiding in plants. Moss helps provide extra moisture and humidity. Hiding Place/Den Provide plenty of plants – either artificial or real – for gargoyle geckos as they need places to hide. Wilmette Pet Center 625 Green Bay Road, Wilmette 847-251-6750 Page 1 of 2 www.wilmettepetcenter.com Updated 4.2019 Cage Type Ten gallon aquariums or critter cages with screen tops work well for gargoyle geckos. -

RVC Exotics Service CRESTED GECKO CARE
RVC Exotics Service Royal Veterinary College Royal College Street London NW1 0TU T: 0207 554 3528 F: 0207 388 8124 www.rvc.ac.uk/BSAH CRESTED GECKO CARE The Crested gecko (Rhacodactylus ciliatus) originates from the islands of New Caledonia where the species was believed to be extinct until 1994 when it was subsequently rediscovered. In its natural environment, it can be found resting in rainforests, sleeping in trunk hollows or leaf litter, and only becomes active at night. Similar to other geckos, it can shed its tail as a defence mechanism (autotomy), but unlike others it lacks an ability to regenerate the tail, so care should be taken while handling. Geckos may live 10-15 years if looked after correctly. HOUSING • As large a vivarium as possible should be provided to enable room for exercise, and a thermal gradient to be created along the length of the tank (hot to cold). Wooden or fibreglass vivaria are ideal as this provides the lizard with some visual security and ventilation can be provided at lizard level. • Good ventilation is required and additional ventilation holes may need to be created. • Hides are required to provide some security. Artificial plants, cardboard boxes, plant pots, logs or commercially available hides can be used. They should be placed both at the warm and cooler ends of the tank. One hide should contain damp moss, kitchen towel or vermiculite to provide a humid environment for shedding. • Substrates suitable for housing lizards include newspaper, Astroturf and some of the commercially available substrates. It is important that the substrates either cannot be eaten, or if they are, do not cause blockages as this can prove fatal. -

A New Locality for Correlophus Ciliatus and Rhacodactylus Leachianus (Sauria: Diplodactylidae) from Néhoué River, Northern New Caledonia
Herpetology Notes, volume 8: 553-555 (2015) (published online on 06 December 2015) A new locality for Correlophus ciliatus and Rhacodactylus leachianus (Sauria: Diplodactylidae) from Néhoué River, northern New Caledonia Mickaël Sanchez1, Jean-Jérôme Cassan2 and Thomas Duval3,* Giant geckos from New Caledonia (Pacific Ocean) We observed seven native gecko species: Bavayia are charismatic nocturnal lizards. This paraphyletic (aff.) cyclura (n=1), Bavayia (aff.) exsuccida (n=1), group is represented by three genera, Rhacodactylus, Correlophus ciliatus (n=1), Dierrogekko nehoueensis Correlophus and Mniarogekko, all endemic to Bauer, Jackman, Sadlier and Whitaker, 2006 (n=1), New Caledonia (Bauer et al., 2012). Rhacodactylus Eurydactylodes agricolae Henkel and Böhme, 2001 leachianus (Cuvier, 1829) is largely distributed on the (n=1), Mniarogekko jalu Bauer, Whitaker, Sadlier and Grande Terre including the Île des Pins and its satellite Jackman, 2012 (n=1) and Rhacodactylus leachianus islands, whereas Correlophus ciliatus Guichenot, 1866 (n=1). Also, the alien Hemidactylus frenatus Dumeril is mostly known in the southern part of the Grande and Bibron, 1836 (n=3) has been sighted. The occurrence Terre, the Île des Pins and its satellite islands (Bauer of C. ciliatus and R. leachianus (Fig. 2 and 3) represent et al., 2012). Here, we report a new locality for both new records for this site. Both gecko species were species in the north-western part of Grande Terre, along observed close to the ground, at a height of less than the Néhoué River (Fig. 1). 1.5 m. The Néhoué River is characterized by gallery forests It is the first time that R. leachianus is recorded in the growing on deep alluvial soils. -

Species Boundaries, Biogeography, and Intra-Archipelago Genetic Variation Within the Emoia Samoensis Species Group in the Vanuatu Archipelago and Oceania" (2008)
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2008 Species boundaries, biogeography, and intra- archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania Alison Madeline Hamilton Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Hamilton, Alison Madeline, "Species boundaries, biogeography, and intra-archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania" (2008). LSU Doctoral Dissertations. 3940. https://digitalcommons.lsu.edu/gradschool_dissertations/3940 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. SPECIES BOUNDARIES, BIOGEOGRAPHY, AND INTRA-ARCHIPELAGO GENETIC VARIATION WITHIN THE EMOIA SAMOENSIS SPECIES GROUP IN THE VANUATU ARCHIPELAGO AND OCEANIA A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Alison M. Hamilton B.A., Simon’s Rock College of Bard, 1993 M.S., University of Florida, 2000 December 2008 ACKNOWLEDGMENTS I thank my graduate advisor, Dr. Christopher C. Austin, for sharing his enthusiasm for reptile diversity in Oceania with me, and for encouraging me to pursue research in Vanuatu. His knowledge of the logistics of conducting research in the Pacific has been invaluable to me during this process. -

Gargoyle Gecko (Rhacodactylus Auriculatus)
©ReptiFiles® — Where Better Reptile Care Begins Gargoyle Gecko (Rhacodactylus auriculatus) Difficulty: Easy Gargoyle geckos are medium-sized 6-8” long lizards that range from cream to dark brown in color with a variety of patterns depending on morph (a reptile that has been bred for a specific color and/or pattern). They have wide toes that give them the ability to climb up smooth surfaces, and a fringe that runs from above their eyes down their back. Gargoyle geckos are native to New Caledonia, a group of islands between Fiji and Australia. They are most commonly found on the islands of Grande Terre and the Isle of Pines. These islands feature a tropical rainforest habitat, where gargoyle geckos can be found among the trees and vines. Gargoyle geckos have a 15-20 year lifespan when cared for properly, and may live longer in some cases. Due to their manageable size, relatively simple care requirements, and tolerance toward humans, gargoyle geckos are popular first-time reptiles. Shopping List Front-opening 18” x 18” x 24” glass terrarium 40w white incandescent heat bulb 5.5” dome lamp with ceramic socket Plug-in lamp dimmer 5-6% T8 UVB bulb, 12” 14” T8 fluorescent hood, with reflector Plug-in light timer Infrared thermometer Pressure sprayer Digital thermometer/hygrometer 2-4” naturalistic substrate Live or artificial plants Vines Branches Magnetic feeding ledge Small gecko feeding cups Crested gecko diet powder Calcium supplement w/o D3 Housing Although gargoyle geckos are small, they still need an enclosure that is large enough to give them adequate opportunity to explore, hunt, and generally exercise natural behaviors. -

Phylogenetic Analysis of the Scaling of Wet and Dry Biological Fibrillar Adhesives
Phylogenetic analysis of the scaling of wet and dry biological fibrillar adhesives A. M. Peattie* and R. J. Full Department of Integrative Biology, University of California, Berkeley, CA 94720-3140 Edited by John G. Hildebrand, University of Arizona, Tucson, AZ, and approved September 26, 2007 (received for review August 10, 2007) Fibrillar, or ‘‘hairy,’’ adhesives have evolved multiple times inde- estimate the pull-off force (Fc) of a field of n hemispherical pendently within arthropods and reptiles. These adhesives exhibit contact elements with diameter s in contact with a smooth highly desirable properties for dynamic attachment, including surface to be orientation dependence, wear resistance, and self-cleaning. Our understanding of how these properties are related to their fibrillar 3 F ϭ n s␥, [1] structure is limited, although theoretical models from the literature c 4 have generated useful hypotheses. We survey the morphology of 81 species with fibrillar adhesives to test the hypothesis that where ␥ is the adhesion energy per area. Contacts are square- packing density of contact elements should increase with body packed, and their diameter is inversely proportional to the size, whereas the size of the contact elements should decrease. We square root of contact density. It is assumed that adhesive pad test this hypothesis in a phylogenetic context to avoid treating area per unit mass decreases with size according to geometric historically related species as statistically independent data points. similarity. This mass-specific decrease is predicted to drive a We find that fiber morphology is better predicted by evolutionary compensatory increase in contact density and a corresponding history and adhesive mechanism than by body size. -

Fauna of Australia 2A
FAUNA of AUSTRALIA 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA Mark N. Hutchinson & Stephen C. Donnellan 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA This review summarises the current hypotheses of the origin, antiquity and history of the order Squamata, the dominant living reptile group which comprises the lizards, snakes and worm-lizards. The primary concern here is with the broad relationships and origins of the major taxa rather than with local distributional or phylogenetic patterns within Australia. In our review of the phylogenetic hypotheses, where possible we refer principally to data sets that have been analysed by cladistic methods. Analyses based on anatomical morphological data sets are integrated with the results of karyotypic and biochemical data sets. A persistent theme of this chapter is that for most families there are few cladistically analysed morphological data, and karyotypic or biochemical data sets are limited or unavailable. Biogeographic study, especially historical biogeography, cannot proceed unless both phylogenetic data are available for the taxa and geological data are available for the physical environment. Again, the reader will find that geological data are very uncertain regarding the degree and timing of the isolation of the Australian continent from Asia and Antarctica. In most cases, therefore, conclusions should be regarded very cautiously. The number of squamate families in Australia is low. Five of approximately fifteen lizard families and five or six of eleven snake families occur in the region; amphisbaenians are absent. Opinions vary concerning the actual number of families recognised in the Australian fauna, depending on whether the Pygopodidae are regarded as distinct from the Gekkonidae, and whether sea snakes, Hydrophiidae and Laticaudidae, are recognised as separate from the Elapidae. -

A Revision of the New Caledonian Genera
Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2, & ANTHONY H. WHITAKER3 1 Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2 Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Short Title: Rhacodactylus Revision Corresponding Author: Aaron M. Bauer Department of Biology, Villanova University 800 Lancaster Avenue, Villanova, Pennsylvania 19085-1699 Phone: 1-610-519-4857 Fax: 1-610-519-7863 email: [email protected] Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2, & ANTHONY H. WHITAKER3 1 Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2 Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Abstract We employed a molecular phylogenetic approach using the mitochondrial ND2 gene and five associated tRNAs (tryptophan, alanine, asparagine, cysteine, tyrosine) and the nuclear RAG1 gene to investigate relationships within the diplodactylid geckos of New Caledonia and particularly among the giant geckos, Rhacodactylus, a charismatic group of lizards that are extremely popular among herpetoculturalists. -

Molecular Evidence for Gondwanan Origins of Multiple Lineages Within A
Journal of Biogeography (J. Biogeogr.) (2009) 36, 2044–2055 ORIGINAL Molecular evidence for Gondwanan ARTICLE origins of multiple lineages within a diverse Australasian gecko radiation Paul M. Oliver1,2* and Kate L. Sanders1 1Centre for Evolutionary Biology and ABSTRACT Biodiversity, University of Adelaide and Aim Gondwanan lineages are a prominent component of the Australian 2Terrestrial Vertebrates, South Australian Museum, North Terrace, Adelaide, SA, terrestrial biota. However, most squamate (lizard and snake) lineages in Australia Australia appear to be derived from relatively recent dispersal from Asia (< 30 Ma) and in situ diversification, subsequent to the isolation of Australia from other Gondwanan landmasses. We test the hypothesis that the Australian radiation of diplodactyloid geckos (families Carphodactylidae, Diplodactylidae and Pygopodidae), in contrast to other endemic squamate groups, has a Gondwanan origin and comprises multiple lineages that originated before the separation of Australia from Antarctica. Location Australasia. Methods Bayesian (beast) and penalized likelihood rate smoothing (PLRS) (r8s) molecular dating methods and two long nuclear DNA sequences (RAG-1 and c-mos) were used to estimate a timeframe for divergence events among 18 genera and 30 species of Australian diplodactyloids. Results At least five lineages of Australian diplodactyloid geckos are estimated to have originated > 34 Ma (pre-Oligocene) and basal splits among the Australian diplodactyloids occurred c. 70 Ma. However, most extant generic and intergeneric diversity within diplodactyloid lineages appears to post-date the late Oligocene (< 30 Ma). Main conclusions Basal divergences within the diplodactyloids significantly pre-date the final break-up of East Gondwana, indicating that the group is one of the most ancient extant endemic vertebrate radiations east of Wallace’s Line. -

Revision of the Giant Geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus)
Zootaxa 3404: 1–52 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2 & ANTHONY H. WHITAKER3 1Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Abstract We employed a molecular phylogenetic approach using the mitochondrial ND2 gene and five associated tRNAs (tryptophan, alanine, asparagine, cysteine, tyrosine) and the nuclear RAG1 gene to investigate relationships within the diplodactylid geckos of New Caledonia and particularly among the giant geckos, Rhacodactylus, a charismatic group of lizards that are extremely popular among herpetoculturalists. The current generic allocation of species within New Caledonian diplodactylids does not adequately reflect their phylogenetic relationships. Bavayia madjo, a high-elevation endemic is not closely related to other Bavayia or to members of any other genus and is placed in a new genus, Paniegekko gen. nov. Rhacodactylus is not monophyletic. The small-bodied and highly autapomorphic genus Eurydactylodes is embedded within Rhacodactylus as sister to R. chahoua. Rhacodactylus ciliatus and R. sarasinorum are sister taxa but are not part of the same clade as other giant geckos and the generic name Correlophus Guichenot is resurrected for them. -

Rules Amending Title 4
Rules Amending Title 4 Hawaii Administrative Rules September 26, 2017 1. Chapter 71 of Title 4, Hawaii Administrative Rules, entitled “Plant and Non-Domestic Animal Quarantine Non-Domestic Animal Import Rules” is amended and compiled to read as follows: “HAWAII ADMINISTRATIVE RULES” TITLE 4 DEPARTMENT OF AGRICULTURE SUBTITLE 6 DIVISION OF PLANT INDUSTRY CHAPTER 71 PLANT AND NON-DOMESTIC ANIMAL QUARANTINE NON-DOMESTIC ANIMAL IMPORT RULES Subchapter 1 General Provisions §4-71-1 Objective §4-71-2 Definitions §4-71-3 Permits §4-71-3.1 User permit fees 71-1 §4-71-4 Submission of permit application to the board §4-71-4.1 Maximum time period for permit approvals, disapprovals, extensions, or automatic approvals §4-71-4.2 Public input and notification for listing §4-71-4.3 Violations Subchapter 2 Non-Domestic Animal Introductions §4-71-5 Notice of quarantine §4-71-6 Prohibited introductions §4-71-6.1 Ad hoc panel for identification of prohibited hybrid animal §4-71-6.5 Permitted introductions §4-71-7 Bond for certain animals §4-71-8 Bonding procedure §4-71-9 Conditions for bonding §4-71-10 Failure to comply with bond conditions Historical note: Chapter 71 is based substantially upon Regulation 2 entitled "Concerning the Introduction of Feral and Other Non-Domestic Animals into Hawaii," of the Division of Entomology and Marketing, Department of Agriculture and Conservation [Eff. 12/12/41; am and ren. Regulation 2 8/30/47; am 9/16/60; R 7/13/81]; and Regulation 3 entitled "Concerning the Introduction of Bacteria, Fungi and Viruses into Hawaii," of the Division of Entomology, Board of Commissioners of Agriculture and Forestry [Eff. -
![1 §4-71-6.5 List of Restricted Animals [ ] Part A: For](https://docslib.b-cdn.net/cover/5559/1-%C2%A74-71-6-5-list-of-restricted-animals-part-a-for-2725559.webp)
1 §4-71-6.5 List of Restricted Animals [ ] Part A: For
§4-71-6.5 LIST OF RESTRICTED ANIMALS [ ] PART A: FOR RESEARCH AND EXHIBITION SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Hirudinea ORDER Gnathobdellida FAMILY Hirudinidae Hirudo medicinalis leech, medicinal ORDER Rhynchobdellae FAMILY Glossiphoniidae Helobdella triserialis leech, small snail CLASS Oligochaeta ORDER Haplotaxida FAMILY Euchytraeidae Enchytraeidae (all species in worm, white family) FAMILY Eudrilidae Helodrilus foetidus earthworm FAMILY Lumbricidae Lumbricus terrestris earthworm Allophora (all species in genus) earthworm CLASS Polychaeta ORDER Phyllodocida FAMILY Nereidae Nereis japonica lugworm PHYLUM Arthropoda CLASS Arachnida ORDER Acari FAMILY Phytoseiidae 1 RESTRICTED ANIMAL LIST (Part A) §4-71-6.5 SCIENTIFIC NAME COMMON NAME Iphiseius degenerans predator, spider mite Mesoseiulus longipes predator, spider mite Mesoseiulus macropilis predator, spider mite Neoseiulus californicus predator, spider mite Neoseiulus longispinosus predator, spider mite Typhlodromus occidentalis mite, western predatory FAMILY Tetranychidae Tetranychus lintearius biocontrol agent, gorse CLASS Crustacea ORDER Amphipoda FAMILY Hyalidae Parhyale hawaiensis amphipod, marine ORDER Anomura FAMILY Porcellanidae Petrolisthes cabrolloi crab, porcelain Petrolisthes cinctipes crab, porcelain Petrolisthes elongatus crab, porcelain Petrolisthes eriomerus crab, porcelain Petrolisthes gracilis crab, porcelain Petrolisthes granulosus crab, porcelain Petrolisthes japonicus crab, porcelain Petrolisthes laevigatus crab, porcelain Petrolisthes