Thermoregulation and Thermal Performance of Crested Geckos (Correlophus Ciliatus) Suggest an Extended Optimality Hypothesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
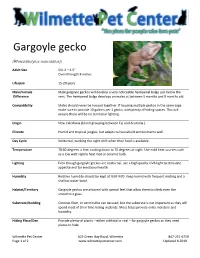
Gargoyle Gecko
Gargoyle gecko (Rhacodactylus auriculatus)) Adult Size SVL 4 – 4.5” Overall length 8 inches Lifespan 15-20 years Male/Female Male gargoyle geckos will develop a very noticeable hemipenal bulge just below the Difference vent. The hemipenal bulge develops on males at between 5 months and 9 months old. Compatibility Males should never be housed together. If housing multiple geckos in the same cage make sure to provide 10 gallons per 1 gecko, with plenty of hiding spaces. This will ensure there will be no territorial fighting. Origin New Caledonia (Island grouping between Fiji and Australia.) Climate Humid and tropical jungles, but adapts to household environments well. Day Cycle Nocturnal, working the night shift when their food is available. Temperature 78-82 degrees is fine, cooling down to 70 degrees at night. Use mild heat sources such as a low watt reptile heat mat or ceramic bulb. Lighting Even though gargoyle geckos are nocturnal, use a high quality UVA light to stimulate appetite and for emotional health. Humidity Relative humidity should be kept at %50-%70. Keep humid with frequent misting and a shallow water bowl. Habitat/Territory Gargoyle geckos are arboreal with special feet that allow them to climb even the smoothest glass. Substrate/Bedding Coconut fiber, or vermiculite can be used, but the substrate is not important as they will spend most of their time hiding in plants. Moss helps provide extra moisture and humidity. Hiding Place/Den Provide plenty of plants – either artificial or real – for gargoyle geckos as they need places to hide. Wilmette Pet Center 625 Green Bay Road, Wilmette 847-251-6750 Page 1 of 2 www.wilmettepetcenter.com Updated 4.2019 Cage Type Ten gallon aquariums or critter cages with screen tops work well for gargoyle geckos. -

RVC Exotics Service CRESTED GECKO CARE
RVC Exotics Service Royal Veterinary College Royal College Street London NW1 0TU T: 0207 554 3528 F: 0207 388 8124 www.rvc.ac.uk/BSAH CRESTED GECKO CARE The Crested gecko (Rhacodactylus ciliatus) originates from the islands of New Caledonia where the species was believed to be extinct until 1994 when it was subsequently rediscovered. In its natural environment, it can be found resting in rainforests, sleeping in trunk hollows or leaf litter, and only becomes active at night. Similar to other geckos, it can shed its tail as a defence mechanism (autotomy), but unlike others it lacks an ability to regenerate the tail, so care should be taken while handling. Geckos may live 10-15 years if looked after correctly. HOUSING • As large a vivarium as possible should be provided to enable room for exercise, and a thermal gradient to be created along the length of the tank (hot to cold). Wooden or fibreglass vivaria are ideal as this provides the lizard with some visual security and ventilation can be provided at lizard level. • Good ventilation is required and additional ventilation holes may need to be created. • Hides are required to provide some security. Artificial plants, cardboard boxes, plant pots, logs or commercially available hides can be used. They should be placed both at the warm and cooler ends of the tank. One hide should contain damp moss, kitchen towel or vermiculite to provide a humid environment for shedding. • Substrates suitable for housing lizards include newspaper, Astroturf and some of the commercially available substrates. It is important that the substrates either cannot be eaten, or if they are, do not cause blockages as this can prove fatal. -

A New Locality for Correlophus Ciliatus and Rhacodactylus Leachianus (Sauria: Diplodactylidae) from Néhoué River, Northern New Caledonia
Herpetology Notes, volume 8: 553-555 (2015) (published online on 06 December 2015) A new locality for Correlophus ciliatus and Rhacodactylus leachianus (Sauria: Diplodactylidae) from Néhoué River, northern New Caledonia Mickaël Sanchez1, Jean-Jérôme Cassan2 and Thomas Duval3,* Giant geckos from New Caledonia (Pacific Ocean) We observed seven native gecko species: Bavayia are charismatic nocturnal lizards. This paraphyletic (aff.) cyclura (n=1), Bavayia (aff.) exsuccida (n=1), group is represented by three genera, Rhacodactylus, Correlophus ciliatus (n=1), Dierrogekko nehoueensis Correlophus and Mniarogekko, all endemic to Bauer, Jackman, Sadlier and Whitaker, 2006 (n=1), New Caledonia (Bauer et al., 2012). Rhacodactylus Eurydactylodes agricolae Henkel and Böhme, 2001 leachianus (Cuvier, 1829) is largely distributed on the (n=1), Mniarogekko jalu Bauer, Whitaker, Sadlier and Grande Terre including the Île des Pins and its satellite Jackman, 2012 (n=1) and Rhacodactylus leachianus islands, whereas Correlophus ciliatus Guichenot, 1866 (n=1). Also, the alien Hemidactylus frenatus Dumeril is mostly known in the southern part of the Grande and Bibron, 1836 (n=3) has been sighted. The occurrence Terre, the Île des Pins and its satellite islands (Bauer of C. ciliatus and R. leachianus (Fig. 2 and 3) represent et al., 2012). Here, we report a new locality for both new records for this site. Both gecko species were species in the north-western part of Grande Terre, along observed close to the ground, at a height of less than the Néhoué River (Fig. 1). 1.5 m. The Néhoué River is characterized by gallery forests It is the first time that R. leachianus is recorded in the growing on deep alluvial soils. -

Thermal Ecology and Habitat Utilization of Rhacodactylus Leachianus from New Caledonia (Squamata: Diplodactylidae)
SALAMANDRA 54(2)Thermal117–122 ecology and15 Mayhabitat 2018 utilizationISSN of 0036–3375 Rhacodactylus leachianus from New Caledonia Thermal ecology and habitat utilization of Rhacodactylus leachianus from New Caledonia (Squamata: Diplodactylidae) Peter Sound1, Friedrich Wilhelm Henkel2, Christian Langner3 & Alfred Seitz1 1) Zoologisches Institut, Abteilung V Populationsbiologie, Universität Mainz, Saarstr. 21, 55099 Mainz, Germany 2) Frielinger Weg 25a, 59174 Kamen, Germany 3) Alstätte 23, 48727 Billerbeck, Germany Corresponding author: Peter Sound, e-mail: [email protected] Received: 24 March 2015 Accepted: 6 February 2018 by Stefan Lötters Abstract. Five specimens (2 ♀, 3 ♂) of the gecko species Rhacodactylus leachianus were radio-tracked for 14 days on Île de Bayonnaise, New Caledonia. Using thermo-sensitive transmitters, specimens were mapped continuously every hour. All movements, behavioural expressions and core temperatures were manually recorded, while data loggers simultaneously recorded the temperatures of microhabitat structures. In an additional trial, all geckos captured on the island were marked with Passive Integrated Transponders (PITs): Twenty-two adults (13 ♀, 9 ♂) and one subadult were marked in total. The mapping of R. leachianus on this island was repeated during 2005, 2007 and 2013. Average core temperatures of 23.4°C (♀) and 24.1°C (♂) were recorded ranging of 18.2–32.0°C (♀) and 18.0–32.2°C (♂), respectively. No sex-dependent differences were noted, but a clear relation to the local air temperature was found. Gravid females showed higher core temperatures (by up to 3°C) during oviposition. The test for equality of variances showed differences between the core temperatures of both sexes and the air temperature, indicating the ability of both sexes for active thermoregulation. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Gargoyle Gecko (Rhacodactylus Auriculatus)
©ReptiFiles® — Where Better Reptile Care Begins Gargoyle Gecko (Rhacodactylus auriculatus) Difficulty: Easy Gargoyle geckos are medium-sized 6-8” long lizards that range from cream to dark brown in color with a variety of patterns depending on morph (a reptile that has been bred for a specific color and/or pattern). They have wide toes that give them the ability to climb up smooth surfaces, and a fringe that runs from above their eyes down their back. Gargoyle geckos are native to New Caledonia, a group of islands between Fiji and Australia. They are most commonly found on the islands of Grande Terre and the Isle of Pines. These islands feature a tropical rainforest habitat, where gargoyle geckos can be found among the trees and vines. Gargoyle geckos have a 15-20 year lifespan when cared for properly, and may live longer in some cases. Due to their manageable size, relatively simple care requirements, and tolerance toward humans, gargoyle geckos are popular first-time reptiles. Shopping List Front-opening 18” x 18” x 24” glass terrarium 40w white incandescent heat bulb 5.5” dome lamp with ceramic socket Plug-in lamp dimmer 5-6% T8 UVB bulb, 12” 14” T8 fluorescent hood, with reflector Plug-in light timer Infrared thermometer Pressure sprayer Digital thermometer/hygrometer 2-4” naturalistic substrate Live or artificial plants Vines Branches Magnetic feeding ledge Small gecko feeding cups Crested gecko diet powder Calcium supplement w/o D3 Housing Although gargoyle geckos are small, they still need an enclosure that is large enough to give them adequate opportunity to explore, hunt, and generally exercise natural behaviors. -

Fauna of Australia 2A
FAUNA of AUSTRALIA 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA Mark N. Hutchinson & Stephen C. Donnellan 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA This review summarises the current hypotheses of the origin, antiquity and history of the order Squamata, the dominant living reptile group which comprises the lizards, snakes and worm-lizards. The primary concern here is with the broad relationships and origins of the major taxa rather than with local distributional or phylogenetic patterns within Australia. In our review of the phylogenetic hypotheses, where possible we refer principally to data sets that have been analysed by cladistic methods. Analyses based on anatomical morphological data sets are integrated with the results of karyotypic and biochemical data sets. A persistent theme of this chapter is that for most families there are few cladistically analysed morphological data, and karyotypic or biochemical data sets are limited or unavailable. Biogeographic study, especially historical biogeography, cannot proceed unless both phylogenetic data are available for the taxa and geological data are available for the physical environment. Again, the reader will find that geological data are very uncertain regarding the degree and timing of the isolation of the Australian continent from Asia and Antarctica. In most cases, therefore, conclusions should be regarded very cautiously. The number of squamate families in Australia is low. Five of approximately fifteen lizard families and five or six of eleven snake families occur in the region; amphisbaenians are absent. Opinions vary concerning the actual number of families recognised in the Australian fauna, depending on whether the Pygopodidae are regarded as distinct from the Gekkonidae, and whether sea snakes, Hydrophiidae and Laticaudidae, are recognised as separate from the Elapidae. -

Molecular Evidence for Gondwanan Origins of Multiple Lineages Within A
Journal of Biogeography (J. Biogeogr.) (2009) 36, 2044–2055 ORIGINAL Molecular evidence for Gondwanan ARTICLE origins of multiple lineages within a diverse Australasian gecko radiation Paul M. Oliver1,2* and Kate L. Sanders1 1Centre for Evolutionary Biology and ABSTRACT Biodiversity, University of Adelaide and Aim Gondwanan lineages are a prominent component of the Australian 2Terrestrial Vertebrates, South Australian Museum, North Terrace, Adelaide, SA, terrestrial biota. However, most squamate (lizard and snake) lineages in Australia Australia appear to be derived from relatively recent dispersal from Asia (< 30 Ma) and in situ diversification, subsequent to the isolation of Australia from other Gondwanan landmasses. We test the hypothesis that the Australian radiation of diplodactyloid geckos (families Carphodactylidae, Diplodactylidae and Pygopodidae), in contrast to other endemic squamate groups, has a Gondwanan origin and comprises multiple lineages that originated before the separation of Australia from Antarctica. Location Australasia. Methods Bayesian (beast) and penalized likelihood rate smoothing (PLRS) (r8s) molecular dating methods and two long nuclear DNA sequences (RAG-1 and c-mos) were used to estimate a timeframe for divergence events among 18 genera and 30 species of Australian diplodactyloids. Results At least five lineages of Australian diplodactyloid geckos are estimated to have originated > 34 Ma (pre-Oligocene) and basal splits among the Australian diplodactyloids occurred c. 70 Ma. However, most extant generic and intergeneric diversity within diplodactyloid lineages appears to post-date the late Oligocene (< 30 Ma). Main conclusions Basal divergences within the diplodactyloids significantly pre-date the final break-up of East Gondwana, indicating that the group is one of the most ancient extant endemic vertebrate radiations east of Wallace’s Line. -

Revision of the Giant Geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus)
Zootaxa 3404: 1–52 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2 & ANTHONY H. WHITAKER3 1Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Abstract We employed a molecular phylogenetic approach using the mitochondrial ND2 gene and five associated tRNAs (tryptophan, alanine, asparagine, cysteine, tyrosine) and the nuclear RAG1 gene to investigate relationships within the diplodactylid geckos of New Caledonia and particularly among the giant geckos, Rhacodactylus, a charismatic group of lizards that are extremely popular among herpetoculturalists. The current generic allocation of species within New Caledonian diplodactylids does not adequately reflect their phylogenetic relationships. Bavayia madjo, a high-elevation endemic is not closely related to other Bavayia or to members of any other genus and is placed in a new genus, Paniegekko gen. nov. Rhacodactylus is not monophyletic. The small-bodied and highly autapomorphic genus Eurydactylodes is embedded within Rhacodactylus as sister to R. chahoua. Rhacodactylus ciliatus and R. sarasinorum are sister taxa but are not part of the same clade as other giant geckos and the generic name Correlophus Guichenot is resurrected for them. -

Gargoyle Gecko Rhacodactylus Auriculatus Care Sheet
Gargoyle Gecko Rhacodactylus auriculatus Care Sheet www.thetdi.com Average Size 6 - 7 inches long Average Lifespan 15 - 20 years Diet Gargoyle Geckos are omnivores. The most commonly used food for Gargoyle Geckos is Repashy Superfood’s Crested Gecko Diet, which is a powder that contains vitamins, minerals, protein, bee pollen, spirulina and other nutritious foods for Gargoyle Geckos. Gargoyle Gecko diets can also be supplemented with a variety of live insects including crickets, mealworms, waxworms, silkworms, tomato hornworms, and cockroaches. Feeding Constant access to the Crested Gecko diet should be available. The diet can be left in the enclosure for 2-3 days before changing as long as it is kept moist. Live insects can be fed once a week as a supplement and should be dusted with calcium powder for each feeding. Housing Habitat - Gargoyle Geckos come from New Caledonia. The environment should consist of diagonal and horizontal branches for perching as well as foliage for hiding. Cork bark rounds are often desired as hiding places. Gargoyle Geckos can be cannibalistic but may be kept in groups. If housed together geckos should be of similar size to avoid injury. Two females generally get along well. A male and female will likely breed if housed together. Two males will become territorial and fight. Gargoyle Geckos do best when housed alone. Size - An adult must have a minimum cage size of 20” Long x 10” Deep x 12” High, also known as a 10-gallon tank. A screen lid is recommended for safety. Substrate - Due to humidity requirements an absorbent substrate is desired. -

Key Innovations and Island Colonization As Engines of Evolutionary Diversification: a Comparative Test with the Australasian Diplodactyloid Geckos
doi: 10.1111/jeb.12261 Key innovations and island colonization as engines of evolutionary diversification: a comparative test with the Australasian diplodactyloid geckos J. GARCIA-PORTA* & T. J. ORD† *Institute of Evolutionary Biology (CSIC-Universitat Pompeu Fabra), Passeig Marıtim de la Barceloneta, Barcelona, Spain †Evolution and Ecology Research Centre, and the School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney NSW, Australia Keywords: Abstract adaptive radiation; The acquisition of key innovations and the invasion of new areas constitute body size disparity; two major processes that facilitate ecological opportunity and subsequent evolutionary rate; evolutionary diversification. Using a major lizard radiation as a model, the lizard; Australasian diplodactyloid geckos, we explored the effects of two key padless; innovations (adhesive toepads and a snake-like phenotype) and the invasion snakelike phenotype; of new environments (island colonization) in promoting the evolution of toepads. phenotypic and species diversity. We found no evidence that toepads had significantly increased evolutionary diversification, which challenges the common assumption that the evolution of toepads has been responsible for the extensive radiation of geckos. In contrast, a snakelike phenotype was associated with increased rates of body size evolution and, to a lesser extent, species diversification. However, the clearest impact on evolutionary diversi- fication has been the colonization of New Zealand and New Caledonia, which were associated with increased rates of both body size evolution and species diversification. This highlights that colonizing new environments can drive adaptive diversification in conjunction or independently of the evolution of a key innovation. Studies wishing to confirm the putative link between a key innovation and subsequent evolutionary diversification must therefore show that it has been the acquisition of an innovation specifically, not the colonization of new areas more generally, that has prompted diversification. -

De Couverture Obligatoire
VETAGRO SUP CAMPUS VETERINAIRE DE LYON Année 2017 - Thèse n°074 BIOLOGIE, ENTRETIEN ET MALADIES LIEES AUX CONDITIONS D’ELEVAGE CHEZ LES PRINCIPAUX REPTILES DE COMPAGNIE PRESENTS EN FRANCE THESE Présentée à l’UNIVERSITE CLAUDE-BERNARD - LYON I (Médecine - Pharmacie) et soutenue publiquement le 16 novembre 2017 pour obtenir le grade de Docteur Vétérinaire par BLANC-REYNAUD Sarah Née le 16 janvier 1993 à Grenoble (38) VETAGRO SUP CAMPUS VETERINAIRE DE LYON Année 2017 - Thèse n°074 BIOLOGIE, ENTRETIEN ET MALADIES LIEES AUX CONDITIONS D’ELEVAGE CHEZ LES PRINCIPAUX REPTILES DE COMPAGNIE PRESENTS EN FRANCE THESE Présentée à l’UNIVERSITE CLAUDE-BERNARD - LYON I (Médecine - Pharmacie) et soutenue publiquement le 16 novembre 2017 pour obtenir le grade de Docteur Vétérinaire par BLANC-REYNAUD Sarah Née le 16 janvier 1993 à Grenoble (38) Liste des Enseignants du Campus Vétérinaire de Lyon Mise à jour le 13/04/2017 3 Remerciements À Monsieur le Professeur Laurent FANTON Professeur à la Faculté de Médecine de Lyon Pour nous avoir fait l’honneur d’accepter de présider notre jury de thèse, Qu’il trouve ici l’expression de nos hommages respectueux. À Madame le Docteur Marie-Pierre CALLAIT-CARDINAL Maître de conférences à VetAgro Sup, Campus Vétérinaire de Lyon Pour nous avoir encadrée tout au long de la rédaction de ce travail, Pour sa bienveillance et ses conseils avisés, Qu’elle reçoive l’expression de notre profonde reconnaissance. À Monsieur le Docteur Antonin TORTEREAU Maître de conférences à VetAgro Sup, Campus Vétérinaire de Lyon Pour avoir accepté de faire partie de notre jury de thèse, Pour avoir pris le temps de relire attentivement notre travail, Sincères remerciements.