Phylogenetic Analysis of the Scaling of Wet and Dry Biological Fibrillar Adhesives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Locality for Correlophus Ciliatus and Rhacodactylus Leachianus (Sauria: Diplodactylidae) from Néhoué River, Northern New Caledonia
Herpetology Notes, volume 8: 553-555 (2015) (published online on 06 December 2015) A new locality for Correlophus ciliatus and Rhacodactylus leachianus (Sauria: Diplodactylidae) from Néhoué River, northern New Caledonia Mickaël Sanchez1, Jean-Jérôme Cassan2 and Thomas Duval3,* Giant geckos from New Caledonia (Pacific Ocean) We observed seven native gecko species: Bavayia are charismatic nocturnal lizards. This paraphyletic (aff.) cyclura (n=1), Bavayia (aff.) exsuccida (n=1), group is represented by three genera, Rhacodactylus, Correlophus ciliatus (n=1), Dierrogekko nehoueensis Correlophus and Mniarogekko, all endemic to Bauer, Jackman, Sadlier and Whitaker, 2006 (n=1), New Caledonia (Bauer et al., 2012). Rhacodactylus Eurydactylodes agricolae Henkel and Böhme, 2001 leachianus (Cuvier, 1829) is largely distributed on the (n=1), Mniarogekko jalu Bauer, Whitaker, Sadlier and Grande Terre including the Île des Pins and its satellite Jackman, 2012 (n=1) and Rhacodactylus leachianus islands, whereas Correlophus ciliatus Guichenot, 1866 (n=1). Also, the alien Hemidactylus frenatus Dumeril is mostly known in the southern part of the Grande and Bibron, 1836 (n=3) has been sighted. The occurrence Terre, the Île des Pins and its satellite islands (Bauer of C. ciliatus and R. leachianus (Fig. 2 and 3) represent et al., 2012). Here, we report a new locality for both new records for this site. Both gecko species were species in the north-western part of Grande Terre, along observed close to the ground, at a height of less than the Néhoué River (Fig. 1). 1.5 m. The Néhoué River is characterized by gallery forests It is the first time that R. leachianus is recorded in the growing on deep alluvial soils. -

Thermal Ecology and Habitat Utilization of Rhacodactylus Leachianus from New Caledonia (Squamata: Diplodactylidae)
SALAMANDRA 54(2)Thermal117–122 ecology and15 Mayhabitat 2018 utilizationISSN of 0036–3375 Rhacodactylus leachianus from New Caledonia Thermal ecology and habitat utilization of Rhacodactylus leachianus from New Caledonia (Squamata: Diplodactylidae) Peter Sound1, Friedrich Wilhelm Henkel2, Christian Langner3 & Alfred Seitz1 1) Zoologisches Institut, Abteilung V Populationsbiologie, Universität Mainz, Saarstr. 21, 55099 Mainz, Germany 2) Frielinger Weg 25a, 59174 Kamen, Germany 3) Alstätte 23, 48727 Billerbeck, Germany Corresponding author: Peter Sound, e-mail: [email protected] Received: 24 March 2015 Accepted: 6 February 2018 by Stefan Lötters Abstract. Five specimens (2 ♀, 3 ♂) of the gecko species Rhacodactylus leachianus were radio-tracked for 14 days on Île de Bayonnaise, New Caledonia. Using thermo-sensitive transmitters, specimens were mapped continuously every hour. All movements, behavioural expressions and core temperatures were manually recorded, while data loggers simultaneously recorded the temperatures of microhabitat structures. In an additional trial, all geckos captured on the island were marked with Passive Integrated Transponders (PITs): Twenty-two adults (13 ♀, 9 ♂) and one subadult were marked in total. The mapping of R. leachianus on this island was repeated during 2005, 2007 and 2013. Average core temperatures of 23.4°C (♀) and 24.1°C (♂) were recorded ranging of 18.2–32.0°C (♀) and 18.0–32.2°C (♂), respectively. No sex-dependent differences were noted, but a clear relation to the local air temperature was found. Gravid females showed higher core temperatures (by up to 3°C) during oviposition. The test for equality of variances showed differences between the core temperatures of both sexes and the air temperature, indicating the ability of both sexes for active thermoregulation. -

Species Boundaries, Biogeography, and Intra-Archipelago Genetic Variation Within the Emoia Samoensis Species Group in the Vanuatu Archipelago and Oceania" (2008)
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2008 Species boundaries, biogeography, and intra- archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania Alison Madeline Hamilton Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Hamilton, Alison Madeline, "Species boundaries, biogeography, and intra-archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania" (2008). LSU Doctoral Dissertations. 3940. https://digitalcommons.lsu.edu/gradschool_dissertations/3940 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. SPECIES BOUNDARIES, BIOGEOGRAPHY, AND INTRA-ARCHIPELAGO GENETIC VARIATION WITHIN THE EMOIA SAMOENSIS SPECIES GROUP IN THE VANUATU ARCHIPELAGO AND OCEANIA A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Alison M. Hamilton B.A., Simon’s Rock College of Bard, 1993 M.S., University of Florida, 2000 December 2008 ACKNOWLEDGMENTS I thank my graduate advisor, Dr. Christopher C. Austin, for sharing his enthusiasm for reptile diversity in Oceania with me, and for encouraging me to pursue research in Vanuatu. His knowledge of the logistics of conducting research in the Pacific has been invaluable to me during this process. -

A Revision of the New Caledonian Genera
Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2, & ANTHONY H. WHITAKER3 1 Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2 Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Short Title: Rhacodactylus Revision Corresponding Author: Aaron M. Bauer Department of Biology, Villanova University 800 Lancaster Avenue, Villanova, Pennsylvania 19085-1699 Phone: 1-610-519-4857 Fax: 1-610-519-7863 email: [email protected] Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2, & ANTHONY H. WHITAKER3 1 Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2 Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Abstract We employed a molecular phylogenetic approach using the mitochondrial ND2 gene and five associated tRNAs (tryptophan, alanine, asparagine, cysteine, tyrosine) and the nuclear RAG1 gene to investigate relationships within the diplodactylid geckos of New Caledonia and particularly among the giant geckos, Rhacodactylus, a charismatic group of lizards that are extremely popular among herpetoculturalists. -

Revision of the Giant Geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus)
Zootaxa 3404: 1–52 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) Revision of the giant geckos of New Caledonia (Reptilia: Diplodactylidae: Rhacodactylus) AARON M. BAUER1,4, TODD R. JACKMAN1, ROSS A. SADLIER2 & ANTHONY H. WHITAKER3 1Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. E-mail: [email protected]; [email protected] 2Department of Herpetology, Australian Museum, 6 College Street, Sydney 2010, New South Wales, Australia. E-mail: [email protected] 3Whitaker Consultants, 270 Thorpe-Orinoco Road, Orinoco, R.D. 1, Motueka 7196, New Zealand. E-mail: [email protected] 4Corresponding author Abstract We employed a molecular phylogenetic approach using the mitochondrial ND2 gene and five associated tRNAs (tryptophan, alanine, asparagine, cysteine, tyrosine) and the nuclear RAG1 gene to investigate relationships within the diplodactylid geckos of New Caledonia and particularly among the giant geckos, Rhacodactylus, a charismatic group of lizards that are extremely popular among herpetoculturalists. The current generic allocation of species within New Caledonian diplodactylids does not adequately reflect their phylogenetic relationships. Bavayia madjo, a high-elevation endemic is not closely related to other Bavayia or to members of any other genus and is placed in a new genus, Paniegekko gen. nov. Rhacodactylus is not monophyletic. The small-bodied and highly autapomorphic genus Eurydactylodes is embedded within Rhacodactylus as sister to R. chahoua. Rhacodactylus ciliatus and R. sarasinorum are sister taxa but are not part of the same clade as other giant geckos and the generic name Correlophus Guichenot is resurrected for them. -

Rules Amending Title 4
Rules Amending Title 4 Hawaii Administrative Rules September 26, 2017 1. Chapter 71 of Title 4, Hawaii Administrative Rules, entitled “Plant and Non-Domestic Animal Quarantine Non-Domestic Animal Import Rules” is amended and compiled to read as follows: “HAWAII ADMINISTRATIVE RULES” TITLE 4 DEPARTMENT OF AGRICULTURE SUBTITLE 6 DIVISION OF PLANT INDUSTRY CHAPTER 71 PLANT AND NON-DOMESTIC ANIMAL QUARANTINE NON-DOMESTIC ANIMAL IMPORT RULES Subchapter 1 General Provisions §4-71-1 Objective §4-71-2 Definitions §4-71-3 Permits §4-71-3.1 User permit fees 71-1 §4-71-4 Submission of permit application to the board §4-71-4.1 Maximum time period for permit approvals, disapprovals, extensions, or automatic approvals §4-71-4.2 Public input and notification for listing §4-71-4.3 Violations Subchapter 2 Non-Domestic Animal Introductions §4-71-5 Notice of quarantine §4-71-6 Prohibited introductions §4-71-6.1 Ad hoc panel for identification of prohibited hybrid animal §4-71-6.5 Permitted introductions §4-71-7 Bond for certain animals §4-71-8 Bonding procedure §4-71-9 Conditions for bonding §4-71-10 Failure to comply with bond conditions Historical note: Chapter 71 is based substantially upon Regulation 2 entitled "Concerning the Introduction of Feral and Other Non-Domestic Animals into Hawaii," of the Division of Entomology and Marketing, Department of Agriculture and Conservation [Eff. 12/12/41; am and ren. Regulation 2 8/30/47; am 9/16/60; R 7/13/81]; and Regulation 3 entitled "Concerning the Introduction of Bacteria, Fungi and Viruses into Hawaii," of the Division of Entomology, Board of Commissioners of Agriculture and Forestry [Eff. -
![1 §4-71-6.5 List of Restricted Animals [ ] Part A: For](https://docslib.b-cdn.net/cover/5559/1-%C2%A74-71-6-5-list-of-restricted-animals-part-a-for-2725559.webp)
1 §4-71-6.5 List of Restricted Animals [ ] Part A: For
§4-71-6.5 LIST OF RESTRICTED ANIMALS [ ] PART A: FOR RESEARCH AND EXHIBITION SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Hirudinea ORDER Gnathobdellida FAMILY Hirudinidae Hirudo medicinalis leech, medicinal ORDER Rhynchobdellae FAMILY Glossiphoniidae Helobdella triserialis leech, small snail CLASS Oligochaeta ORDER Haplotaxida FAMILY Euchytraeidae Enchytraeidae (all species in worm, white family) FAMILY Eudrilidae Helodrilus foetidus earthworm FAMILY Lumbricidae Lumbricus terrestris earthworm Allophora (all species in genus) earthworm CLASS Polychaeta ORDER Phyllodocida FAMILY Nereidae Nereis japonica lugworm PHYLUM Arthropoda CLASS Arachnida ORDER Acari FAMILY Phytoseiidae 1 RESTRICTED ANIMAL LIST (Part A) §4-71-6.5 SCIENTIFIC NAME COMMON NAME Iphiseius degenerans predator, spider mite Mesoseiulus longipes predator, spider mite Mesoseiulus macropilis predator, spider mite Neoseiulus californicus predator, spider mite Neoseiulus longispinosus predator, spider mite Typhlodromus occidentalis mite, western predatory FAMILY Tetranychidae Tetranychus lintearius biocontrol agent, gorse CLASS Crustacea ORDER Amphipoda FAMILY Hyalidae Parhyale hawaiensis amphipod, marine ORDER Anomura FAMILY Porcellanidae Petrolisthes cabrolloi crab, porcelain Petrolisthes cinctipes crab, porcelain Petrolisthes elongatus crab, porcelain Petrolisthes eriomerus crab, porcelain Petrolisthes gracilis crab, porcelain Petrolisthes granulosus crab, porcelain Petrolisthes japonicus crab, porcelain Petrolisthes laevigatus crab, porcelain Petrolisthes -
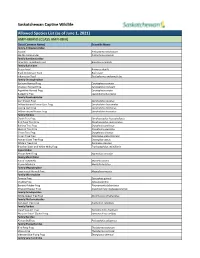
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Invasive Rodents, an Overlooked Threat for Skinks in a Tropical Island Hotspot of Biodiversity Martin Thibault, Fabrice Brescia, Herve Jourdan, Eric Vidal
Invasive rodents, an overlooked threat for skinks in a tropical island hotspot of biodiversity Martin Thibault, Fabrice Brescia, Herve Jourdan, Eric Vidal To cite this version: Martin Thibault, Fabrice Brescia, Herve Jourdan, Eric Vidal. Invasive rodents, an overlooked threat for skinks in a tropical island hotspot of biodiversity. New Zealand Journal of Ecology, The New Zealand Ecological Society, 2017, 41 (1), pp.74-83. 10.20417/nzjecol.41.9. hal-01681556 HAL Id: hal-01681556 https://hal.archives-ouvertes.fr/hal-01681556 Submitted on 11 May 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 74 Invasive rodents, an overlooked threat for skinks in a tropical island hotspot of biodiversity Martin Thibault1,2*, Fabrice Brescia2, Hervé Jourdan1 and Eric Vidal1 1Aix Marseille Univ, Univ Avignon, CNRS, IRD, IMBE, Centre IRD Nouméa - BP A5, 98848 Nouméa Cedex, New Caledonia 2Institut Agronomique Néo-Calédonien (IAC), “Diversités biologique et fonctionnelle des écosystèmes terrestres”, BP 73, 98890 Païta, New Caledonia *Author for correspondence: (Email: [email protected]) Abstract: Squamata are one of the most threatened groups among island vertebrates, facing high pressure from exotic species. However, the contribution of small terrestrial reptiles in invasive rodents’ diet remains poorly investigated, partly because of the lack of tools for accurately identifying chewed prey fragments in gut contents. -

Phd Thesis, University of Wollongong
Comparative phylogeography and diversity of Australian Monsoonal Tropics lizards Rebecca Jan Laver ORCID ID 0000-0002-6319-7213 Doctor of Philosophy January 2017 The School of BioSciences Faculty of Science The University of Melbourne Submitted in total fulfilment of the requirements of the degree of Doctor of Philosophy Produced on archival quality paper Thesis Abstract Tropical savannah biomes cover ~20% of the world’s landmass, however the biodiversity encompassed within these environments and the underlying processes that have shaped it remain poorly understood. Recent increased research to address this knowledge gap have begun to reveal surprisingly high amounts of deep, geographically- structured diversity, much of which is cryptic or hidden within morphologically similar species complexes. These patterns are especially emphasized in vertebrate taxa which are intrinsically linked to rock escarpments and ranges that dissect the savannah woodlands and grasslands of many of these biomes, hinting at a role of heterogeneous topography in structuring diversity. The remote Australian Monsoonal Tropics (AMT) spanning the north of the Australian continent is a particularly vast, and relatively undisturbed, tropical savannah region. Recent increased surveys are revealing numerous new species and endemism hotspots, indicating we are only just beginning to uncover the true biodiversity levels within this biome. Not only is there a relative paucity of knowledge regarding the present diversity within this region, but there is also limited understanding of how this diversity came to be. Phylogeographic studies can assist us in establishing current patterns of diversity and their evolutionary significance within regions and biomes. Furthermore, by comparing and contrasting the patterns and timing of diversification within and between biomes for multiple ecologically diverse taxa, we can begin to elucidate the history of these biomes and the environmental processes that have shaped the diversity we observe today. -

Full Account (PDF)
FULL ACCOUNT FOR: Felis catus Felis catus System: Terrestrial Kingdom Phylum Class Order Family Animalia Chordata Mammalia Carnivora Felidae Common name cat (English), domestic cat (English), pusiniveikau (English, Fiji), house cat (English), Hauskatze (German), poti (Maori), feral cat (English) Synonym Similar species Summary Felis catus was domesticated in the eastern Mediterranean c. 3000 years ago. Considering the extent to which cats are valued as pets, it is not surprising that they have since been translocated by humans to almost all parts of the world. Notable predators, cats threaten native birdlife and other fauna, especially on islands where native species have evolved in relative isolation from predators. view this species on IUCN Red List Species Description Felis catus is a small animal in the wild (up to 5kg, but more commonly 1.5 -3.0kg) but may be considerably heavier when domesticated. Colour is extremely variable in domesticated varieties and feral cats commonly revert to black, tabby or tortoiseshell with varying extents of white starting from the belly and breast. Lifecycle Stages Gestation: 65 days. Weaning: 35-40 days. Sexual maturity: 9 months. Global Invasive Species Database (GISD) 2021. Species profile Felis catus. Available Pag. 1 from: http://www.iucngisd.org/gisd/species.php?sc=24 [Accessed 07 October 2021] FULL ACCOUNT FOR: Felis catus Habitat Description Feral cats adapt to a variety of habitat types and circumstances. On the Australian continent they inhabit forests and woodland habitats in eastern, western and northern parts of the country (Dickman 1996). On Hahajima Island, Japan, feral cats have been observed widely in various kinds of habitats, including primary forests (Kawakami and Higuchi 2002). -

Etude Naturaliste Et Terrariophile Sur Le Genre Rhacodactylus FITZINGER 1843 Version Word Photos
1 2 Droits d’Auteur La présente étude ,qu’elle soit issue du forum Les Dragons d’Asgard ou diffusée par courrier électronique sous forme de fichier pdf imprimable ,demeure la stricte propriété de son auteur et est déposée à la CNIL . En conséquence ,toute reproduction partielle ou totale des textes ,photos et images tombe sous la loi du copyright et nécessite au préalable l’accord écrit de l’auteur .Toute diffusion non autorisée est passible de poursuites pénales .Les acheteurs ou bénéficiaires à titre gratuit de la version pdf sont priés de ne pas diffuser cette étude de quelque manière que ce soit ,de ne pas en faire de multiples copies partielles ou totales ,et de ne pas en diffuser des extraits de quelque manière que ce soit sans permission .Ce que vous avez sous les yeux représente un travail de rédaction ,de synthèse d’informations et de démarches pour les autorisations pour les matériaux communiqués à l’auteur qui correspond à plusieurs centaines d’heures de travail .Il vous est donc demandé de respecter ce travail et de ne pas le diffuser .Tout préjudice moral ou financier résultant d’une utilisation non autorisée fera l’objet de poursuites . Le prix public de cette étude éditée à compte d’auteur est de 10,00 € sous forme de fichier pdf imprimable en couleur . Toute demande devra être adressée à l’auteur uniquement par courrier électronique : [email protected] This study is protected by copyright laws and self-published by its author through his Internet forum Les Dragons d’Asgard and on a pdf colour printable version (orders