Black Hawii 0085O 10729.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Species of Deepwater Cardinalfish from the Indo-Pacific
Ichthyol Res DOI 10.1007/s10228-013-0352-0 FULL PAPER Two new species of deepwater cardinalfish from the Indo-Pacific, with a definition of the Epigonus pandionis group (Perciformes: Epigonidae) Makoto Okamoto • Hiroyuki Motomura Received: 29 March 2013 / Revised: 9 May 2013 / Accepted: 9 May 2013 Ó The Ichthyological Society of Japan 2013 Abstract Two new Indo-Pacific species of deepwater diameter 14.5–15.4 % SL; and lower-jaw length cardinalfish, Epigonus lifouensis and E. tuberculatus are 16.0–17.6 % SL. A key to the species and some comments described based on the specimens collected from the on the group are provided based on examination of all Loyalty Islands and Cocos-Keeling Islands, respectively. members (nine species, including two new species) of the These species belong to the Epigonus pandionis group group. defined as lacking an opercular spine, having more than 43 pored lateral-line scales to the end of the hypural and dorsal- Keywords Deepwater cardinal fish Á Epigonus Á fin rays VII-I, 9–11. Epigonus lifouensis is distinguished New Caledonia Á Australia from other members of the group by a combination of the following characters: ribs present on the last abdominal vertebra; tongue toothless; tubercle absent on inner sym- Introduction physis of lower jaw; eye elliptical; total gill rakers 24–25; pectoral-fin rays 18–19; pyloric caeca 10–13; body depth Fishes of the genus Epigonus Rafinesque 1810 (deepwater 17.0–17.1 % SL; and posterior half of oral cavity and ton- cardinalfish) are distributed from temperate to tropical waters gue black. Epigonus tuberculatus is distinguished from in the world (Mayer 1974;Okamoto2012; Okamoto and other members of the group by a combination of the fol- Motomura 2012). -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Epigonus Okamotoi, a New Species of Deepwater Cardinalfish from New Britain, Papua New Guinea, Solomon Sea, Western Pacific Ocean (Teleostei: Epigonidae)
FishTaxa (2017) 2(3): 116-122 E-ISSN: 2458-942X Journal homepage: www.fishtaxa.com © 2017 FISHTAXA. All rights reserved Epigonus okamotoi, a new species of deepwater cardinalfish from New Britain, Papua New Guinea, Solomon Sea, western Pacific Ocean (Teleostei: Epigonidae) Ronald FRICKE Im Ramstal 76, 97922 Lauda-Königshofen, Germany. Corresponding author: *E-mail: [email protected] Abstract A new species of deepwater cardinalfish, Epigonus okamotoi from off southwestern New Britain, Papua New Guinea, is described on the basis of a single specimen collected with a trawl in 315-624 m depth in Ainto Bay. The new species is characterised by the following characters: dorsal-fin rays VII+I, 9; pectoral-fin rays 15; total gill rakers 22; pyloric caeca 4; pored lateral-line scales 47+4; scales below lateral line 8; vertebrae 10+15; opercular spine present; maxillary mustache-like process absent; ribs absent on last abdominal vertebra; upper margin of pectoral-fin base on level or upper margin of pupil; proximal radial of first anal-fin pterygiophore slender; mouth cavity light grey. The new species is compared with other species in the genus. Keywords: Deepwater cardinalfishes, New species, Identification key, New Guinea. Zoobank: urn:lsid:zoobank.org:pub:B565A437-DA54-4AF9-B0F0-87126CE665F6 urn:lsid:zoobank.org:act:9B04EE8C-BD17-4849-B541-112F9F46B9B5 Introduction The deepwater cardinalfishes of the family Epigonidae Poey 1861 are a group of bathydemersal marine fishes occurring on the continental and island slopes of all tropical and warm temperate oceans (Okamoto et al. 2011; Laan et al. 2014; Nelson et al. 2016), at depths of 100 to more than 1,000 m. -

Global Progress in Ecosystem-Based Fisheries Management
26th Lowell Wakefield Fisheries Symposium Global Progress in Ecosystem-Based Fisheries Management editors gordon h. kruse • howard i. browman • kevern l. cochrane diana evans • glen s. jamieson • patricia a. livingston douglas woodby • chang ik zhang university of alaska fairbanks Global Progress in Ecosystem-Based Fisheries Management editors gordon h. kruse • howard i. browman • kevern l. cochrane diana evans • glen s. jamieson • patricia a. livingston douglas woodby • chang ik zhang Alaska university of alaska fairbanks Elmer E. Rasmuson Library Cataloging in Publication Data: Global progress in ecosystem-based fisheries management / editors : G.H. Kruse … [et al.] – Fairbanks, Alaska : Alaska Sea Grant College Program, University of Alaska Fairbanks, 2012. p. : ill. ; cm. – (Alaska Sea Grant College Program, University of Alaska Fairbanks ; AK-SG-12-01) Proceedings of the symposium Ecosystems 2010 : global progress on ecosystem- based fisheries management, November 8-11, 2010, Anchorage, Alaska. Includes bibliographical references. 1. Fishery management—Congresses. 2. Sustainable fisheries—Congresses. 3. Marine ecosystem management—Congresses. I. Title. II. Kruse, Gordon H. III. Series: Lowell Wakefield Fisheries symposia series (26th : 2010 : Anchorage, Alaska). IV. Series: Alaska Sea Grant College Program report ; AK-SG-12-01. SH329.S89 P76 2012 ISBN 978-1-56612-166-8 doi:10.4027/gpebfm.2012 Citation Kruse, G.H., H.I. Browman, K.L. Cochrane, D. Evans, G.S. Jamieson, P.A. Livingston, D. Woodby, and C.I. Zhang, eds. 2012. Global Progress in Ecosystem-Based Fisheries Management. Alaska Sea Grant, University of Alaska Fairbanks. Credits This book is published by Alaska Sea Grant, supported by the U.S. Department of Commerce, NOAA National Sea Grant Office, grant NA10OAR4170097, project A/161- 02, and by the University of Alaska Fairbanks with state funds. -

Epigonus Telescopus (Risso, 1810)
Epigonus telescopus (Risso, 1810) AphiaID: 126858 BLACK CARDINAL FISH Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Perciformes (Ordem) > Percoidei (Subordem) > Epigonidae (Familia) Heessen, Henk Heessen, Henk Sinónimos Epigonus macrophthalmus Rafinesque, 1810 Pomatomus cuvieri Cocco, 1829 Pomatomus cuvierii Cocco, 1829 Pomatomus telescopium Risso, 1810 Pomatomus telescopus Risso, 1810 Referências MARTINS, R.; CARNEIRO, M., 2018. Manual de identificação de peixes ósseos da costa continental portuguesa – Principais Características Diagnosticantes. IPMA, I.P., 204p de Morais, L., Smith-Vaniz, W.F., Carpenter, K.E. & de Bruyne, G. 2015. Epigonus telescopus. The IUCN Red List of Threatened Species 2015: e.T198653A15547631. http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T198653A15547631.en. additional source Risso, A. (1810). Ichthyologie de Nice ou histoire naturelle des poissons du 1 département des Alpes-Maritimes. Schoell, Paris., available online at https://doi.org/10.5962/bhl.title.7052 [details] additional source Wheeler, A. (1992). A list of the common and scientific names of fishes of the British Isles. J. Fish Biol. 41(Suppl. A): 1-37 [details] additional source Froese, R. & D. Pauly (Editors). (2018). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; Eschmeyer, W.N.; Froese, R. (2001). Pisces, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. -

Mediterranean Sea
OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi MEDITERRANEAN The IUCN Red List of Threatened Species™ – Regional Assessment OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi The IUCN Red List of Threatened Species™ – Regional Assessment Compilers: Dania Abdul Malak Mediterranean Species Programme, IUCN Centre for Mediterranean Cooperation, calle Marie Curie 22, 29590 Campanillas (Parque Tecnológico de Andalucía), Málaga, Spain Suzanne R. Livingstone Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, c/o Conservation International, Arlington, VA 22202, USA David Pollard Applied Marine Conservation Ecology, 7/86 Darling Street, Balmain East, New South Wales 2041, Australia; Research Associate, Department of Ichthyology, Australian Museum, Sydney, Australia Beth A. Polidoro Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Annabelle Cuttelod Red List Unit, IUCN Species Programme, 219c Huntingdon Road, Cambridge CB3 0DL,UK Michel Bariche Biology Departement, American University of Beirut, Beirut, Lebanon Murat Bilecenoglu Department of Biology, Faculty of Arts and Sciences, Adnan Menderes University, 09010 Aydin, Turkey Kent E. Carpenter Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Bruce B. -

2018 Population of Grey Gurnard In
Population biology of grey gurnard (Eutrigla gurnardus L.; Triglidae) in the ANGOR UNIVERSITY coastal waters of Northwest Wales McCarthy, Ian; Cant, James; Marriott, Andrew Journal of Applied Ichthyology DOI: 10.1111/jai.13733 PRIFYSGOL BANGOR / B Published: 01/08/2018 Peer reviewed version Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): McCarthy, I., Cant, J., & Marriott, A. (2018). Population biology of grey gurnard (Eutrigla gurnardus L.; Triglidae) in the coastal waters of Northwest Wales. Journal of Applied Ichthyology, 34(4), 896-905. https://doi.org/10.1111/jai.13733 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 27. Sep. 2021 1 Population biology of grey gurnard (Eutrigla gurnardus L.; Triglidae) in 2 the coastal waters of Northwest Wales 3 4 Running Title: Population biology of grey gurnard 5 6 I. -

Species Profile: Black Cardinalfish
Epigonus telescopus Species Profile SEAFO South East Atlantic Fisheries Organisation (Adapted from www.ictioterm.es) UPDATE DRAFT Ana Rita Vieira and Ivone Figueiredo – 09/2010 (Updated:) 1. Taxomony Phylum Chordata Subphylum Vertebrata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Acanthopterygii Order Perciformes Suborder Percoidei Family Epigonidae Genus Epigonus Rafinesque, 1810 Species Epigonus telescopus (Risso, 1810) Epigonus macrophthalmus Rafinesque, 1810 Synonyms Pomatomus cuvieri Cocco, 1829 Pomatomus telescopus Risso, 1810 Common name Black cardinalfish Species code EPI 2. Species characteristics 2.1 Distribution This species is widely distributed in the North Atlantic (Iceland to the Canary Islands and Corner Seamounts), in the western Mediterranean, in the Southeast Atlantic, Indian and Southwest Pacific (Walvis Ridge off southwestern Africa to New Zealand) (Abramov, 1992). (Adapted from www.fishbase.org) 2.2 Habitat E. telescopus is a bathydemersal fish on continental slope at 75-1200 m, but is most abundant at 300-800 m (Tortonese, 1986). In New Zealand waters, the preferred depth range of schools is 600-900 m (Field et al., 1997). This depth overlaps the upper end of the depth range of orange roughy (Hoplostethus atlanticus ) and the lower end of alfonsino ( Beryx splendens ) and bluenose ( Hyperoglyphe antarctica ) (Field et al., 1997). 2.3 Biological characteristics Morphology Dorsal spines (total): 7- 8; Dorsal soft rays (total): 9-11; Anal spines: 2; Anal soft rays: 9. No opercular spines. Snout blunt, eye large. Mouth large, lower jaw equaling or slightly protruding beyond upper jaw. Pyloric caeca 21-34. Brown-violet or black, iridescent in life (Fishbase, 2010). Maximum size Maximum size reported is 75 cm, for New Zealand specimens (Anon, 2007). -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Age, Growth and Maturity of Tub Gurnard (Chelidonichthys Lucerna
Age, growth and maturity of tub gurnard (Chelidonichthys lucerna ANGOR UNIVERSITY Linnaeus 1758: Triglidae) in the inshore coastal waters of Northwest Wales, UK McCarthy, Ian; Marriott, Andrew Journal of Applied Ichthyology DOI: 10.1111/jai.13614 PRIFYSGOL BANGOR / B Published: 01/06/2018 Peer reviewed version Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): McCarthy, I., & Marriott, A. (2018). Age, growth and maturity of tub gurnard (Chelidonichthys lucerna Linnaeus 1758: Triglidae) in the inshore coastal waters of Northwest Wales, UK. Journal of Applied Ichthyology, 34(3), 581-589. https://doi.org/10.1111/jai.13614 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 28. Sep. 2021 Age, growth and maturity of tub gurnard (Chelidonichthys lucerna ANGOR UNIVERSITY Linnaeus 1758: Triglidae) in the inshore coastal waters of Northwest Wales, UK McCarthy, Ian; Marriott, Andrew Journal of Applied Ichthyology PRIFYSGOL BANGOR / B Accepted/In press: 12/12/2017 Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): McCarthy, I., & Marriott, A. -

Galicia Bank - Bank Galicia the Briefing Aa O Vlae T Iprac Fr the for Importance Level
The Galicia Bank - A Potential MPA Location The Galicia Bank is located at 42° 67’ N and 11° 74’ W about 200 km West of the Galician coast of Spain. Potential Reasons for Selection Seamounts such as the Galicia Bank function like an island within the ocean. Based on their three- dimensional structure, they provide a higher number of microhabitats than the barren surroundings and host a more biodiverse benthic fauna respectively. The intensity of vertical mixing of the water column and thus the primary productivity is often higher around them than in the open ocean. Many fish species and cetaceans tend to aggregate in their vicinity and use them as briefing feeding and spawning grounds. Information about the Galicia Bank and its ecosystem is Fig. 1: Location of the Galicia Bank rather scarce as only very few biological surveys have (after Lallemand et al. 1985) been taken place there. The anthropogenic impact on the To the North, Northwest it slopes very steeply from bank e.g. in terms of fishery has not been adressed yet. approximately 1,000 m down to the abyssal plain at Following the precautionary principle the designation of 5,000 m. It is mainly composed of basaltic lavas and the bank as a MPA under OSPAR would help to preserve uplifted oceanic crust. its biological features and to coordinate any kind of On top of the bank, the sediment primarily consists of human activity in a sustainable manner. Moreover, it shells of pelagic foraminifera while the organic carbon could promote further investigations to adress the actual content is quite low. -
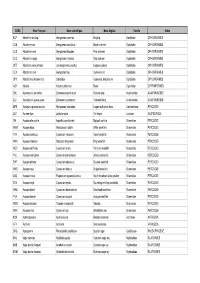
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa