Trophodynamics of Eastern Shelf and Slope SEF Fishery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sound Production by a Recoiling System in the Pempheridae and Terapontidae
JOURNAL OF MORPHOLOGY 00:00–00 (2016) Sound Production by a Recoiling System in the Pempheridae and Terapontidae Eric Parmentier,1* Michael L. Fine,2 and Hin-Kiu Mok3,4 1Laboratoire de Morphologie Fonctionnelle et Evolutive, AFFISH-RC, Institut de Chimie, Bat.^ B6c, Universitede Lie`ge, Lie`ge, B-4000, Belgium 2Department of Biology, Virginia Commonwealth University, Richmond, Virginia 23284-2012 3Department of Oceanography and Asia-Pacific Ocean Research Center, National Sun Yat-sen University, Kaohsiung, 80424, Taiwan 4National Museum of Marine Biology and Aquarium, Checheng, Pingtung, 944, Taiwan ABSTRACT Sound-producing mechanisms in fishes several hundred Hz (Parmentier and Fine, 2016). are extraordinarily diversified. We report here original Two main types of mechanism have been analyzed. mechanisms of three species from two families: the (1) Sound can be produced as a forced response: pempherid Pempheris oualensis, and the terapontids the muscle contraction rate or time for a twitch Terapon jarbua and Pelates quadrilineatus.Allsonic mechanismsarebuiltonthesamestructures.Theros- determines sound fundamental frequency (Sko- tral part of the swimbladder is connected to a pair of glund, 1961; Connaughton, 2004; Fine et al., 2001; large sonic muscles from the head whereas the poste- Onuki and Somiya, 2004; Parmentier et al., 2011, rior part is fused with bony widenings of vertebral 2014). In various catfish species (Boyle et al., bodies. Two bladder regions are separated by a stretch- 2014, 2015), the sonic muscles originate on the able fenestra that allows forward extension of the ante- skull and insert on a bony modified rib (Mullerian€ rior bladder during muscle contraction. A recoiling ramus) that attaches to the swimbladder (Ladich apparatus runs between the inner face of the anterior and Bass, 1996). -

Pacific Plate Biogeography, with Special Reference to Shorefishes
Pacific Plate Biogeography, with Special Reference to Shorefishes VICTOR G. SPRINGER m SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 367 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Dedication Donald Perrin De Sylva
Dedication The Proceedings of the First International Symposium on Mangroves as Fish Habitat are dedicated to the memory of University of Miami Professors Samuel C. Snedaker and Donald Perrin de Sylva. Samuel C. Snedaker Donald Perrin de Sylva (1938–2005) (1929–2004) Professor Samuel Curry Snedaker Our longtime collaborator and dear passed away on March 21, 2005 in friend, University of Miami Professor Yakima, Washington, after an eminent Donald P. de Sylva, passed away in career on the faculty of the University Brooksville, Florida on January 28, of Florida and the University of Miami. 2004. Over the course of his diverse A world authority on mangrove eco- and productive career, he worked systems, he authored numerous books closely with mangrove expert and and publications on topics as diverse colleague Professor Samuel Snedaker as tropical ecology, global climate on relationships between mangrove change, and wetlands and fish communities. Don pollutants made major scientific contributions in marine to this area of research close to home organisms in south and sedi- Florida ments. One and as far of his most afield as enduring Southeast contributions Asia. He to marine sci- was the ences was the world’s publication leading authority on one of the most in 1974 of ecologically important inhabitants of “The ecology coastal mangrove habitats—the great of mangroves” (coauthored with Ariel barracuda. His 1963 book Systematics Lugo), a paper that set the high stan- and Life History of the Great Barracuda dard by which contemporary mangrove continues to be an essential reference ecology continues to be measured. for those interested in the taxonomy, Sam’s studies laid the scientific bases biology, and ecology of this species. -

Vulnerable Marine Ecosystems – Processes and Practices in the High Seas Vulnerable Marine Ecosystems Processes and Practices in the High Seas
ISSN 2070-7010 FAO 595 FISHERIES AND AQUACULTURE TECHNICAL PAPER 595 Vulnerable marine ecosystems – Processes and practices in the high seas Vulnerable marine ecosystems Processes and practices in the high seas This publication, Vulnerable Marine Ecosystems: processes and practices in the high seas, provides regional fisheries management bodies, States, and other interested parties with a summary of existing regional measures to protect vulnerable marine ecosystems from significant adverse impacts caused by deep-sea fisheries using bottom contact gears in the high seas. This publication compiles and summarizes information on the processes and practices of the regional fishery management bodies, with mandates to manage deep-sea fisheries in the high seas, to protect vulnerable marine ecosystems. ISBN 978-92-5-109340-5 ISSN 2070-7010 FAO 9 789251 093405 I5952E/2/03.17 Cover photo credits: Photo descriptions clockwise from top-left: Acanthagorgia spp., Paragorgia arborea, Vase sponges (images courtesy of Fisheries and Oceans, Canada); and Callogorgia spp. (image courtesy of Kirsty Kemp, the Zoological Society of London). FAO FISHERIES AND Vulnerable marine ecosystems AQUACULTURE TECHNICAL Processes and practices in the high seas PAPER 595 Edited by Anthony Thompson FAO Consultant Rome, Italy Jessica Sanders Fisheries Officer FAO Fisheries and Aquaculture Department Rome, Italy Merete Tandstad Fisheries Resources Officer FAO Fisheries and Aquaculture Department Rome, Italy Fabio Carocci Fishery Information Assistant FAO Fisheries and Aquaculture Department Rome, Italy and Jessica Fuller FAO Consultant Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2016 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Mullidae 3175
click for previous page Perciformes: Percoidei: Mullidae 3175 MULLIDAE Goatfishes (surmullets) by J.E. Randall iagnostic characters: Body moderately elongate and somewhat compressed (size to 50 cm). Two Dlong unbranched barbels on chin; mouth low on head, the lower jaw inferior, the cleft slightly oblique; dentition variable but teeth conical, either in villiform bands or in 1 or 2 rows, never as enlarged canines (except in adult males of western Atlantic and eastern Pacific species of Pseudupeneus, the teeth of which are slightly enlarged). A single flat spine posteriorly on opercle (a second less developed spine may be present); margin of preopercle smooth. Two well-separated dorsal fins, the first with VII or VIII (usually VIII) slender spines (first spine often very small), the second fin with 9 soft rays (first unbranched); anal fin with I spine and 6 or 7 soft rays; caudal fin deeply forked, with 13 branched rays; pelvic fins with I spine and 5 soft rays; pectoral fins with 13 to 18 rays. Scales finely ctenoid; head and body completely scaly (except preorbital region of some species of Upeneus). Lateral line complete, following contour of back, the pored scales to base of caudal fin 27 to 38. Colour: ground colour in preservative usually pale, in life often whitish to light red; most species with distinctive black, brown, red, or yellow markings; median fins often with stripes or oblique bands. 2 dorsal fins, 1st with VII-VIII spines, 2nd with 9 soft rays 2 barbels on chin Habitat, biology, and fisheries: Most goatfishes inhabit shallow seas. They are usually found on open sand or mud bottoms, at least for feeding (though the species of Parupeneus and Mulloidichthys are often seen on coral reefs or rocky substrata). -

Appendices Appendices
APPENDICES APPENDICES APPENDIX 1 – PUBLICATIONS SCIENTIFIC PAPERS Aidoo EN, Ute Mueller U, Hyndes GA, and Ryan Braccini M. 2015. Is a global quantitative KL. 2016. The effects of measurement uncertainty assessment of shark populations warranted? on spatial characterisation of recreational fishing Fisheries, 40: 492–501. catch rates. Fisheries Research 181: 1–13. Braccini M. 2016. Experts have different Andrews KR, Williams AJ, Fernandez-Silva I, perceptions of the management and conservation Newman SJ, Copus JM, Wakefield CB, Randall JE, status of sharks. Annals of Marine Biology and and Bowen BW. 2016. Phylogeny of deepwater Research 3: 1012. snappers (Genus Etelis) reveals a cryptic species pair in the Indo-Pacific and Pleistocene invasion of Braccini M, Aires-da-Silva A, and Taylor I. 2016. the Atlantic. Molecular Phylogenetics and Incorporating movement in the modelling of shark Evolution 100: 361-371. and ray population dynamics: approaches and management implications. Reviews in Fish Biology Bellchambers LM, Gaughan D, Wise B, Jackson G, and Fisheries 26: 13–24. and Fletcher WJ. 2016. Adopting Marine Stewardship Council certification of Western Caputi N, de Lestang S, Reid C, Hesp A, and How J. Australian fisheries at a jurisdictional level: the 2015. Maximum economic yield of the western benefits and challenges. Fisheries Research 183: rock lobster fishery of Western Australia after 609-616. moving from effort to quota control. Marine Policy, 51: 452-464. Bellchambers LM, Fisher EA, Harry AV, and Travaille KL. 2016. Identifying potential risks for Charles A, Westlund L, Bartley DM, Fletcher WJ, Marine Stewardship Council assessment and Garcia S, Govan H, and Sanders J. -
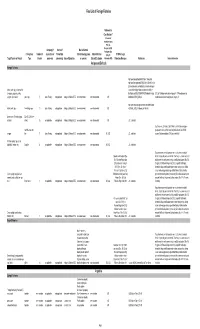
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Morphological Variations in the Scleral Ossicles of 172 Families Of
Zoological Studies 51(8): 1490-1506 (2012) Morphological Variations in the Scleral Ossicles of 172 Families of Actinopterygian Fishes with Notes on their Phylogenetic Implications Hin-kui Mok1 and Shu-Hui Liu2,* 1Institute of Marine Biology and Asia-Pacific Ocean Research Center, National Sun Yat-sen University, Kaohsiung 804, Taiwan 2Institute of Oceanography, National Taiwan University, 1 Roosevelt Road, Sec. 4, Taipei 106, Taiwan (Accepted August 15, 2012) Hin-kui Mok and Shu-Hui Liu (2012) Morphological variations in the scleral ossicles of 172 families of actinopterygian fishes with notes on their phylogenetic implications. Zoological Studies 51(8): 1490-1506. This study reports on (1) variations in the number and position of scleral ossicles in 283 actinopterygian species representing 172 families, (2) the distribution of the morphological variants of these bony elements, (3) the phylogenetic significance of these variations, and (4) a phylogenetic hypothesis relevant to the position of the Callionymoidei, Dactylopteridae, and Syngnathoidei based on these osteological variations. The results suggest that the Callionymoidei (not including the Gobiesocidae), Dactylopteridae, and Syngnathoidei are closely related. This conclusion was based on the apomorphic character state of having only the anterior scleral ossicle. Having only the anterior scleral ossicle should have evolved independently in the Syngnathioidei + Dactylopteridae + Callionymoidei, Gobioidei + Apogonidae, and Pleuronectiformes among the actinopterygians studied in this paper. http://zoolstud.sinica.edu.tw/Journals/51.8/1490.pdf Key words: Scleral ossicle, Actinopterygii, Phylogeny. Scleral ossicles of the teleostome fish eye scleral ossicles and scleral cartilage have received comprise a ring of cartilage supporting the eye little attention. It was not until a recent paper by internally (i.e., the sclerotic ring; Moy-Thomas Franz-Odendaal and Hall (2006) that the homology and Miles 1971). -

Marine Protected Species Identification Guide
Department of Primary Industries and Regional Development Marine protected species identification guide June 2021 Fisheries Occasional Publication No. 129, June 2021. Prepared by K. Travaille and M. Hourston Cover: Hawksbill turtle (Eretmochelys imbricata). Photo: Matthew Pember. Illustrations © R.Swainston/www.anima.net.au Bird images donated by Important disclaimer The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it. Department of Primary Industries and Regional Development Gordon Stephenson House 140 William Street PERTH WA 6000 Telephone: (08) 6551 4444 Website: dpird.wa.gov.au ABN: 18 951 343 745 ISSN: 1447 - 2058 (Print) ISBN: 978-1-877098-22-2 (Print) ISSN: 2206 - 0928 (Online) ISBN: 978-1-877098-23-9 (Online) Copyright © State of Western Australia (Department of Primary Industries and Regional Development), 2021. ii Marine protected species ID guide Contents About this guide �������������������������������������������������������������������������������������������1 Protected species legislation and international agreements 3 Reporting interactions ���������������������������������������������������������������������������������4 Marine mammals �����������������������������������������������������������������������������������������5 Relative size of cetaceans �������������������������������������������������������������������������5