4.2.4 Calculation of Fluxes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tucdirectory Trusted by Your Union
TUCDIRECTORY TRUSTED BY YOUR UNION best practice advice • returning officer statutory ballots • industrial action ballots turnout maximisation • independent scrutineer consultative ballots • data processing and capture secure print and fulfilment • results analysis artwork and design • membership profiling • e-voting e-distribution • branded voting websites digital engagement Contact us: 020 8365 8909 [email protected] @ERSvotes CONTENTS SECTION 1 SECTION 6 INTRODUCTION INTERNATIONAL Welcome 5 International affiliations 94 TUC structure 8 ITUC regional organisations 97 ITUC global union federations 98 SECTION 2 TUC PEOPLE SECTION 7 EXTERNAL CONTACTS Policy staff at Congress House 14 Policy staff in the regions 21 Campaigning, charity 102 and community SECTION 3 Employer and personnel 105 TUC SERVICES organisations Financial and other services 106 Information service 24 Government 106 Publishing 24 Industrial relations, workers’ 108 Websites 24 rights and union history TUC Aid 25 International, environment 109 Organising Academy 26 and anti-poverty Centres for the Unemployed 26 Legal 110 Trades Union Councils 27 Non-TUC unions and 111 TUC and young people 27 confederations Tolpuddle Martyrs Museum 29 Pensions 111 TUC Library Collections 29 Political 112 TUC archive 30 Research organisations 112 and public bodies SECTION 4 Skills and education 116 TRADE UNIONS Union statistics 32 SECTION 8 TUC member unions 41 CALENDAR 118 Confederations of unions 80 SECTION 5 UNIONLEARN AND TUC EDUCATION TUC Education 83 Learning through unions 88 1 SECTION ONE INTRODUCTION WELCOME 5 TUC STRUCTURE 8 WELCOME TO THE 2016 EDITION OF THE TUC DIRECTORY Every membership organisation offers a single, reliable, statistical resource for those who follow its fortunes, and for the TUC that is the Directory, our annual yearbook about our unions and TUC work. -

111 Piccadilly, Manchester, Greater Manchester
111 Piccadilly, Manchester, Greater Manchester View this office online at: https://www.newofficeeurope.com/details/serviced-offices-111-piccadilly-man chester-greater These fantastic serviced offices are well placed with terrific access to the major transport network adjacent to the Piccadilly railway station. After an intense and stylish refurbishment program the centre provides office accommodation of the very highest specification with spacious, contemporary offices and suites, a wonderful welcoming reception area with the whole place flooded by plenty of natural daylight. Offices start from 600 square feet to 4,000 square feet to suit many different business needs with flexible terms from a single year up to 25 years. This truly prestigious location and working environment can do wonders for your corporate image and your productivity. Transport links Nearest railway station: Manchester Piccadilly Nearest road: Nearest airport: Key features 24 hour access Access to multiple centres nation-wide Administrative support AV equipment Board room Car parking spaces Close to railway station Comfortable lounge Company signage Conference rooms Conference rooms Disabled facilities (DDA/ADA compliant) Double glazing Flexible contracts Furnished workspaces High-speed internet (dedicated) Kitchen facilities Lift Meeting rooms Modern interiors Office cleaning service On-site management support Open plan workstations Postal facilities/mail handling Reception staff Secure car parking Security system Staff on site 7 days a week Suspended Ceilings Telephone answering service Town centre location Unbranded offices WC (separate male & female) Wireless networking Location In an unrivaled city center location just a stones throw from Piccadilly railway and tram station and within a short walking distance from Piccadilly Gardens bus station. -

Manchester City Centre Third Edition 1:3,500
Manchester City Centre Third Edition 1:3,500 830 A 831 B 832 C 833 D 834 E 835 F 836 G 837 H 838 J 839 K 840 L 841 M 842 N 843 P 844 Q 845 R 846 S 847 T 848 U 849 V 850 990 VICTORIA STATION APPROACH ANGEL 990 Westminster CANNON Renault FB Arena Point Car Park GMC Fire Service Royal Mail G R Cornerhouse A665 Miller Street LUDGATE B E N D I X S T R E E T E ROLLA ST House Victoria Victoria STREET E Garage Leisuredrive Smithfield Sorting Office B6184 Hotel Car Park T H O M P S O N Training Centre N Eagle MEN Arena Cravans Arena Service Station Braziers Aldridge Inn PCS Station Car Park New Century PH G O U L D E N S T R E E T DRIVE M I L L E R S T R E E T DYCHE STREET Wing Yip Dunlop DAWSON STREET Car Park STREET S T R E E T Thompson Street PH Locksmith NEW MIRABEL STREET W.H.Smith House (Co-op) Beer House A6042 BRIDGEGREENGATE STREET Car Park HODSON ST Greengate Venus MILLGATE Fire Station 1 HUNT'S BANK Ladies Old Bank KENWRIGHT County 1 Cannon Green Court Clothing STREET M A R S H ARecord L L WELLINGTON Samuel Building MAYES STREET COLLIER STREET Crowther CAYGILL STREET Trident STREETOffice STREET WEST KING STREET Greengate WALKER'S Burns Q U E E N BOOND S STREET T R E E T CWS Car Stephen AC LONG Smithfield Car Park Upton Medical House House Dolby LA N E CROFT Redfern Park Project C L O S E B L A C K Autobody HANOVERCentre S S I D Y Hotel Snippers Gents Building ROCHDALE ROAD A Wing Yip Black Friar Car Park C A62 Stuart Repairs Club Addington HATTER PH DUKE STREET A D D I N G T O N Chinese Supermarket 989 House Library REDFERNHolyoake STREET -

Phase I Desk Study and Preliminary Contaminated Land Risk Assessment
Client: Principal Contractor: Trafalgar Leisure Ltd 373 - 375 Station Rd Harrow Middlesex HA1 2AW Phase I Desk Study and Preliminary Contaminated Land Risk Assessment 43 - 47 Piccadilly Gardens, Manchester 5902/PIX PCLRA Phase I Jan 2018 v1.00 Unit 4 Sheffield Street Heaton Norris Stockport Cheshire SK4 1RU Telephone : 0161 480 8885 Jan 2018 Site Ref: 5902/PIX V1.00 PCLRA e-mail : [email protected] 43 - 47 Piccadilly Gardens, Manchester Client: Principal Contractor: Trafalgar Leisure Ltd 373 - 375 Station Rd Harrow Middlesex HA1 2AW Contents Phase I Desk Study and Preliminary Contaminated Land Risk Assessment 1. Introduction ................................................................................................................................ 4 2. Description of the Site ............................................................................................................... 4 3. Historical Researches ................................................................................................................. 5 3.1 Historical Mapping .................................................................................................... 5 3.2 Anecdotal Historical Information ............................................................................ 5 3.3 Previous Intrusive Investigation .............................................................................. 6 3.4 Environmental Data Search ...................................................................................... 6 3.4.1 Historical Land uses .............................................................................................. -

Leyland Historical Society
LEYLAND HISTORICAL SOCIETY (Founded 1968) Registered Charity No. 1024919 PRESIDENT Mr. W. E. Waring CHAIR VICE-CHAIR Mr. P. Houghton Mrs. E. F. Shorrock HONORARY SECRETARY HONORARY TREASURER Mr. M. J. Park Mr. E. Almond Tel: (01772) 337258 AIMS To promote an interest in history generally and that of the Leyland area in particular MEETINGS Held on the first Monday of each month (September to July inclusive) at 7.30 pm in The Shield Room, Banqueting Suite, Civic Centre, West Paddock, Leyland SUBSCRIPTIONS Vice Presidents: £10.00 per annum Members: £10.00 per annum School Members: £1.00 per annum Casual Visitors: £3.00 per meeting A MEMBER OF THE LANCASHIRE LOCAL HISTORY FEDERATION THE HISTORIC SOCIETY OF LANCASHIRE AND CHESHIRE and THE BRITISH ASSOCIATION FOR LOCAL HISTORY Visit the Leyland Historical Society's Web Site at: http//www.leylandhistoricalsociety.co.uk C O N T E N T S Page Title Contributor 4 Editorial Mary Longton 5 Society Affairs Peter Houghton 7 From a Red Letter Day to days with Red Letters Joan Langford 11 Fascinating finds at Haydock Park Edward Almond 15 The Leyland and Farington Mechanics’ Institution Derek Wilkins Joseph Farington: 3rd December 1747 to Joan Langford 19 30th December 1821 ‘We once owned a Brewery’ – W & R Wilkins of Derek Wilkins 26 Longton 34 More wanderings and musings into Memory Lane Sylvia Thompson Railway trip notes – Leyland to Manchester Peter Houghton 38 Piccadilly Can you help with the ‘Industrial Heritage of Editor 52 Leyland’ project? Lailand Chronicle No. 56 Editorial Welcome to the fifty-sixth edition of the Lailand Chronicle. -

Application Number 121014/FO/2018 Date of Appln 20Th Sep 2018
Application Number Date of Appln Committee Date Ward 121014/FO/2018 20th Sep 2018 13th Dec 2018 Piccadilly Ward Proposal Erection of 23 storey building (land at no. 14-16 Piccadilly) plus plant level and conversion of adjacent building (no. 12 Piccadilly) (basement to fourth floor) to create 356 bedroom hotel above ground floor breakfast room and lobby. Location 12 - 16 Piccadilly, Manchester, M1 3AN Applicant Toyoko Inn Co Ltd, C/o Agent, Agent Mrs Emma Jones, GVA HOW Planning, Norfolk House, 7 Norfolk Street, Manchester, M2 5GP Background Planning permission and listed building were granted in December 2007 ( ref no’s 082829 and 082830 ) for the conversion and refurbishment of the 4 storey former Union Bank (12 Piccadilly) a Grade II Listed Building and the erection of a 9 storey building on the adjacent vacant site to provide office accommodation throughout. The building would have been around 40m high. Planning permission and listed building were granted in November 2013 ( ref no’s 103766/FO/2013/C2 and 103769/LO/2013/C2 ) for the conversion of the listed building and the erection of a 20 storey building to create a 258 bedroom hotel with a ground floor bar 117m). These permissions have now expired. However, the principle of development on the site, including a new build hotel of 20 storeys and conversion of the existing listed building into a hotel use has been established. This current application comprises an updated version of the 2013 scheme by the same architect for a different hotel operator. Description of site The site measures 0.07 hectares and is bounded by Piccadilly, Gore Street and Chatham Street with the Waldorf Public House and Indemnity House (no.7 Chatham St) immediately to the rear. -
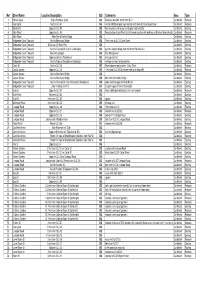
Copy of Bin Spreadsheet
Ref Street Name Location/Description GIS Comments Area Type 1 Pioneer Quay Slip to Rochdale Canal GIS Relocate round bin 10mts from SLC1 Castlefield Relocate 2 Deansgate Next to SLC 01 GIS next to N55 Deansgate sign relocate 3mts towards crossing and road Castlefield Relocate 3 Blantyre St Opposite SLC 04 GIS Post mounted next to pay and display machine 3005 Castlefield Existing 4 Slate Wharf Opposite SLC 04 GIS Relocate from Slate Wharf SLC04 towards junction with walkway on Blantyre Street directly oppositeCastlefield Relocate 5 Slate Wharf Near Marsh Brother Bridge GIS Castlefield Existing 6 Bridgewater Canal Tow path Next to Merchants Bridge GIS 75mts rear of SLC15 Duke Street Castlefield Existing 7 Bridgewater Canal Tow path At the rear Of Wharf Pub GIS Castlefield Existing 8 Bridgewater Canal Tow path Next to Canal basin (rear of Castle Quay) GIS past the wooden bridge from the Wharf Pub 2nd SLC Castlefield Existing 9 Bridgewater Canal Tow path Rear of Castlegate GIS Blue heritage cast Castlefield Existing 10 Bridgewater Canal Tow path Opposite Grocer's Warehouse GIS hertitage cast lid Castlefield Existing 11 Bridgewater Canal Tow path Next to Steps at the bottom of footbridge GIS heritage cast next to two benches Castlefield Existing 12 Castle St Next to SLC 15 GIS Move between two benches - Duke Street Castlefield Relocate 13 Catalan Square Opposite SLC 15 GIS 11mts from SLC15 Duke Street next to wishing well Castlefield Existing 14 Catalan Square Next to Merchants Bridge GIS Castlefield Existing 15 Catalan Square Next to Merchants -
Private Hire Driver & Hackney Carriage
Private Hire Driver & Hackney Carriage Knowledge Test Revision Guide Version 1.2 (updated 06 January 2016) © Manchester City Council. You may NOT reproduce this document in its entirety.manchester.gov.uk Any partial reproduction or alteration is expressly forbidden without the prior permission of the copyright holder. 2 Private Hire Driver & Hackney Carriage Knowledge Test: Revision Guide Private Hire Driver & Hackney Carriage Knowledge Test: Revision Guide 3 Contents Introduction ........................................4 Conditions and Customer Care ........... 27 The Knowledge Test what you need Lists, locations, to pass .................................................5 places and premises ....................... 28 Section 1 City Centre bars, restaurants and Private Hire and Hackney Carriage ....6 private clubs ......................................28 Part 1: Reading and understanding the Hotels ............................................... 32 Greater Manchester A–Z Atlas .............6 Transport interchanges ......................34 Finding a location in the A–Z Hospitals ........................................... 35 Finding ‘St’, ‘Sa’ and ‘Gt’ locations .......... 7 City Centre banks, building societies ..36 Part 2: Places Theatres, libraries and cinemas ..........36 Finding ‘The’ locations..............................8 Exhibition centres, conference Section 2 centres, art galleries and museums .... 37 Private Hire .....................................9 Parks and open spaces ....................... 37 Part 1: Routes from one -

Manchester City Centre Fourth Edition 1:3,500
Manchester City Centre Fourth Edition 1:3,500 X 828 A 829 B 830 C 831 D 832 E 833 F 834 G 835 H 836 J 837 K 838 L 839 M 840 N 841 P 842 Q 843 R 844 S 845 T 846 U 847 V 848 W 849 850 Y 851 Z 852 SAINT MINCING ST HINT 992 BRIDGEW SPRINGFIELD LIVESEY 992 Whitefriar Riverbank Y HALF ST RESERVOIR STREET GREA CHEETHAM HILLRiver Irk C.W.S. MOORHEAD STREET A River ON STREET W Cromech British Gas Court Tower chimney weir T LANE Tobacco MISTLET Engineering SIMON Caroline Brewery Tap Factory G O U L Euro Impex The Friars County House ASPIN LANE STREET STREET STREET SILK STREET TWILLBROOK PH steps Sudell St Primary School DRIVE DEAN ROAD Boddingtons Brewery FB POPLIN DRIVE Rajan FB T Ashton R E E C.W.S. Industrial A T OE GROVE Fashion Clothing Car Park SIMEON STREET STREET TER SENIOR STREET CHANGE DUCIE Entrance House Car Park LUDGA Fordville D Estate ROE STREET 1 BLACKF Reception CROWN LANE S OLD MOUNT 1 Irwell Crown Decorator Bramhall MILLOW ST ASPIN PH CLIVE House SCHOOL ALK Centre Court CARDING GROVE King William FB STREET Entrance PH Thermos Norflex STREET Broughton LANE TE DRIVE The Fourth 6 Crown & ST MIC SQ S STREET BACK SUDELL Tavern STREET NEW BRIDGE STREET HAEL'S NAPLESRagged STREET RIARS 5 Saw Mill STREETSchool T ASHLEY Kays Joinery Cushion SHARP R E E A (SUBWAY) 4 DURANT GLASSHOUSE ST ANS STREET Parkers CIS ASHLEY D FB CLARION chimneySTREET 991 W ROPE ROAD 991 A N EV Road 3 Apartments STYLE STREET Marble ST N AC O McDonalds Printing Car Park Subway Ring STREET 2 HILL Manchester MUNSTER STREET& Stationery ANGEL Arch Inn ST Supa- STREET -
![Foundation Home Loans Solicitor Panel Press [CTRL] ‘F’ to Open the Search Box](https://docslib.b-cdn.net/cover/4890/foundation-home-loans-solicitor-panel-press-ctrl-f-to-open-the-search-box-8534890.webp)
Foundation Home Loans Solicitor Panel Press [CTRL] ‘F’ to Open the Search Box
Foundation Home Loans Solicitor panel Press [CTRL] ‘F’ to open the search box Panel Number Firm First Line Address Second Line Address Postcode PA2929F/SPO Abacus Solicitors LLP 10 Grappenhall Road Stockton Heath WA4 2AG PA2399F/SPO Abacus Solicitors LLP Reedham House 31 King Street West M3 2PN PA6297F/SPO ABC Worcester Limited 37 Foregate Street WR1 1EE PA1015E/SPO Abels 6 College Place SO15 2XL PA1909E/SPO Abensons Law Limited 102 Allerton Road Mossley Hill L18 2DG PA3105F/SPO Aberavon Lawyers Limited 10-11 Courtland Place SA13 1JJ Unit A1, Sovereign Business PA3129F/SPO ABH Law Limited Kings Croft Court WN1 3AP Park PA2076F/SPO Access Law LLP 175-177 Shirley Road SO15 3FG PA1838C/SPO Ackroyd Legal (London) LLP 402-404 Commercial Road E1 0LB PA6301F/SPO Adam Solicitors 1-7 Bent Street M8 8NF PA2276F/SPO Adams & Remers LLP Trinity House School Hill BN7 2NN PA2378F/SPO Adams & Remers LLP Commonwealth House 55-58 Pall Mall SW1Y 5JH PA3112F/SPO Adams Harrison 43 High Street Sawston CB22 3BG PA3110F/SPO Adams Harrison 14-18 Church Street CB10 1JW PA3111F/SPO Adams Harrison 52A High Street CB9 8AR PA3116F/SPO Adie Pepperdine Ltd 3 The Landings Burton Waters LN1 2TU PA6946F/SPO AFG LAW LTD 20 Mawdsley Street BL1 1LE PA6977F/SPO AFG LAW LTD 9 Canon Court Institute Street BL1 1PZ PA6976F/SPO AFG LAW LTD 14A Market Street BL9 0AJ PA5288F/SPO AK Law (London) Limited 15 Spring Bridge Mews W5 2AB PA3123F/SPO Alderson Law LLP 4-8 Stanley Street NE24 2BU PA3122F/SPO Alderson Law LLP Castle Square NE61 1YL PA2952G/SPO Aldridge Brownlee Solicitors LLP -

Bruntwood Annual Review 2020
After a very successful year in 2019, as The costs of lockdown will also be felt in this we gained real momentum in our Works accounting year, as will the ongoing impact on Creating spaces and SciTech divisions, we were buoyant the economy beyond it. That said, the signs are for collaboration heading into 2020… But, of course, our that the Covid crisis has made the Bruntwood Getting back Works and SciTech propositions relatively more world has changed drastically since then. and innovation attractive to companies looking for more from their offices. The impact of COVID has shocked us With the onset of the fourth industrial all, affecting everyone personally and revolution, we’ve become the ‘home’ for to business professionally. We’ve seen friends, family, and Covid - a catalyst science and tech businesses. We’ve seen our colleagues suffer during a crisis unparalleled Bruntwood SciTech customers thrive over the in living memory. Many of us faced the grief of for change? past 12 months, as many played a huge part losing loved ones; the loss of livelihoods and in the efforts in the fight against Covid. The futures; and the mental impact. It will take time If you believed the headlines through the science and technology sectors will be just as for society to regroup and recover... Summer of 2020, it was easy to think the office, important post Covid, in the recovery of our and cities, were dead, but as time has gone economy. Our Bruntwood SciTech campuses We worked quickly to deliver a new strategy. on it has become clearer that this is not the offer that crucial space to enable and facilitate We accelerated the Works and SciTech case. -

111 Piccadilly Manchester’S Smartest Workspace About Bruntwood Works
111 Piccadilly Manchester’s smartest workspace About Bruntwood Works 111 Piccadilly Manchester’s smartest workspace 111 Piccadilly Manchester’s smartest workspace Bruntwood Works Pioneer buildings are the future of workspace design and innovation – the buildings of tomorrow, today. Each site’s forward-thinking spaces offer individuality and flexibility, along with unique events and retail Space to offerings. They create the perfect place for the Bruntwood Works community of vibrant businesses to connect. You’ll find bespoke designs at each location, all based on six key themes - amenity, art, biophilia, sustainability, innovate the wellness and technology. We’re master reinventors, crafting something unique and exciting, mixing the old with the new. All of our Pioneer buildings offer workspace for businesses of all sizes, from a single coworking desk to serviced offices everyday and leased spaces. Six key6 themes of Pioneer Amenity Wellbeing Biophilia Amenity Sustainability Technology 111 Piccadilly Manchester’s smartest workspace One seriously smart space Everything at 111 Piccadilly is tailored to you and your needs. Designed specifically to enhance your everyday by giving you control over your work environment and your wellness, this really is the smartest workspace around. It’s more sustainable too, from the power to the plants. And all of this is supported by the Platinum standard WELL accreditation which will be awarded in 2021. But the innovation doesn’t stop there. Within these walls, and beyond, digital art installations inspire, and community-building events bring pioneering thought leaders together. All this conveniently waiting on the doorstep of Manchester’s Piccadilly Station. And with coworking and leased offices all under one roof, 111 Piccadilly gives you the flexibility to discover or create the workspace that works best for you.