The Taxonomy and Molecular Phylogeny of Potentilla L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Coastal and Marine Ecological Classification Standard (2012)
FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard Marine and Coastal Spatial Data Subcommittee Federal Geographic Data Committee June, 2012 Federal Geographic Data Committee FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard, June 2012 ______________________________________________________________________________________ CONTENTS PAGE 1. Introduction ..................................................................................................................... 1 1.1 Objectives ................................................................................................................ 1 1.2 Need ......................................................................................................................... 2 1.3 Scope ........................................................................................................................ 2 1.4 Application ............................................................................................................... 3 1.5 Relationship to Previous FGDC Standards .............................................................. 4 1.6 Development Procedures ......................................................................................... 5 1.7 Guiding Principles ................................................................................................... 7 1.7.1 Build a Scientifically Sound Ecological Classification .................................... 7 1.7.2 Meet the Needs of a Wide Range of Users ...................................................... -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

San Bruno Mountain Habitat Conservation Plan Year
SAN BRUNO MOUNTAIN HABITAT CONSERVATION PLAN YEAR 2019-20 ACTIVITIES REPORT FOR FEDERALLY LISTED SPECIES Endangered Species 10(a)(1)(B) Permit TE215574-6 Prepared By: Evan Cole San Mateo County Parks Department 455 County Center, 4th Floor Redwood City, CA 94063 December 2020 TABLE OF CONTENTS Table of Contents I. INTRODUCTION ..............................................................................................................7 A. Covered Species Population Status ................................................................................... 7 2019 MISSION BLUE STATUS ......................................................................................................................... 8 2020 CALLIPPE SILVERSPOT STATUS.............................................................................................................. 8 2020 SAN BRUNO ELFIN STATUS ................................................................................................................... 9 RARE PLANT STATUS ..................................................................................................................................... 9 II. STATUS OF SPECIES OF CONCERN .........................................................................9 A. Mission Blue Butterfly (Icaricia icarioides missionensis) .............................................. 9 METHODOLOGY .......................................................................................................................................... 10 RESULTS ..................................................................................................................................................... -

Mountain Plants of Northeastern Utah
MOUNTAIN PLANTS OF NORTHEASTERN UTAH Original booklet and drawings by Berniece A. Andersen and Arthur H. Holmgren Revised May 1996 HG 506 FOREWORD In the original printing, the purpose of this manual was to serve as a guide for students, amateur botanists and anyone interested in the wildflowers of a rather limited geographic area. The intent was to depict and describe over 400 common, conspicuous or beautiful species. In this revision we have tried to maintain the intent and integrity of the original. Scientific names have been updated in accordance with changes in taxonomic thought since the time of the first printing. Some changes have been incorporated in order to make the manual more user-friendly for the beginner. The species are now organized primarily by floral color. We hope that these changes serve to enhance the enjoyment and usefulness of this long-popular manual. We would also like to thank Larry A. Rupp, Extension Horticulture Specialist, for critical review of the draft and for the cover photo. Linda Allen, Assistant Curator, Intermountain Herbarium Donna H. Falkenborg, Extension Editor Utah State University Extension is an affirmative action/equal employment opportunity employer and educational organization. We offer our programs to persons regardless of race, color, national origin, sex, religion, age or disability. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Robert L. Gilliland, Vice-President and Director, Cooperative Extension -

Annotated Checklist of Vascular Flora, Bryce
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 ON THE COVER Matted prickly-phlox (Leptodactylon caespitosum), Bryce Canyon National Park, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Sarah Topp Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 January 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. -
2017 Rule Change Proposal 14
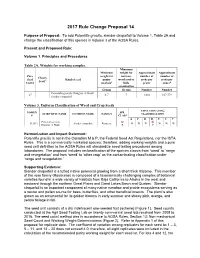
2017 Rule Change Proposal 14 Purpose of Proposal: To add Potentilla gracilis, slender cinquefoil to Volume 1, Table 2A and change the classification of this species in Volume 3 of the AOSA Rules. Present and Proposed Rule: Volume 1. Principles and Procedures Table 2A. Weights for working samples. Minimum Minimum weight for Approximate Approximate Pure weight for noxious- number of number of Chaffy Seed Kind of seed purity weed seed or seeds per seeds per Seed a Unit # analysisb bulk gramc ounced examination Grams Grams Number Number Potentilla gracilis Douglas ex Hook 27 0.7 7 3,802 107,779 slender cinquefoil Volume 3. Uniform Classification of Weed and Crop Seeds CONTAMINATING NOMEN SPP. SCIENTIFIC NAME COMMON NAME FAMILY CLASSIFICATION # CLASS A F H R S T V Potentilla gracilis W W 313631 slender cinquefoil Rosaceae W W W W W W Douglas ex Hook R C Harmonization and Impact Statement: Potentilla gracilis is not in the Canadian M & P, the Federal Seed Act Regulations, nor the ISTA Rules. This is a commercially marketed species; therefore, adding working weights and a pure seed unit definition to the AOSA Rules will standardize seed testing procedures among laboratories. The proposal includes reclassification of the species classis from ‘weed’ to ‘range and revegetation’ and from ‘weed’ to ‘other crop’ as the contaminating classification under ‘range and revegetation.’ Supporting Evidence: Slender cinquefoil is a tufted native perennial growing from a short thick rhizome. This member of the rose family (Rosaceae) is composed of a taxonomically challenging complex of botanical varieties found in a wide variety of habitats from Baja California to Alaska in the west and eastward through the northern Great Plains and Great Lakes Basin and Quebec. -

Floristic Inventory of Subalpine Parks in the Coeur D'alene River Drainage, Northern Idaho
FLORISTIC INVENTORY OF SUBALPINE PARKS IN THE COEUR D'ALENE RIVER DRAINAGE, NORTHERN IDAHO by Robert K. Moseley Conservation Data Center December 1993 Idaho Department of Fish and Game Natural Resource Policy Bureau 600 South Walnut, P.O. Box 25 Boise, Idaho 83707 Jerry M. Conley, Director Cooperative Challenge Cost-share Project Idaho Panhandle National Forests Idaho Department of Fish and Game ABSTRACT Treeless summits and ridges in the otherwise densely forested mountains of northern Idaho, have a relatively unique flora compared with surrounding communities. Although small in area, these subalpine parks add greatly to the biotic diversity of the regional landscape and are habitats for several vascular taxa considered rare in Idaho. I conducted a floristic inventory of 32 parks in the mountains of the Coeur d'Alene River drainage and adjacent portions of the St. Joe drainage. The project is a cooperative one between the Idaho Department of Fish and Game's Conservation Data Center and the Idaho Panhandle National Forest. The subalpine park flora contains 151 taxa representing 97 genera in 34 families. Carex are surprisingly depauperate, in terms of both numbers and cover, as is the alien flora, which is comprised of only three species. I discovered populations of five rare species, including Carex xerantica, which is here reported for Idaho for the first time. Other rare species include Astragalus bourgovii, Carex californica, Ivesia tweedyi, and Romanzoffia sitchensis. Stevens Peak is the highest summit and is phytogeographically unique in the study area. It contains habitat for six taxa occurring nowhere else in the study area, all having high-elevation cordilleran or circumboreal affinities. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

A Natural Resource Condition Assessment for Sequoia and Kings Canyon National Parks Appendix 14 – Plants of Conservation Concern
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science A Natural Resource Condition Assessment for Sequoia and Kings Canyon National Parks Appendix 14 – Plants of Conservation Concern Natural Resource Report NPS/SEKI/ NRR—2013/665.14 In Memory of Rebecca Ciresa Wenk, Botaness ON THE COVER Giant Forest, Sequoia National Park Photography by: Brent Paull A Natural Resource Condition Assessment for Sequoia and Kings Canyon National Parks Appendix 14 – Plants of Conservation Concern Natural Resource Report NPS/SEKI/ NRR—2013/665.14 Ann Huber University of California Berkeley 41043 Grouse Drive Three Rivers, CA 93271 Adrian Das U.S. Geological Survey Western Ecological Research Center, Sequoia-Kings Canyon Field Station 47050 Generals Highway #4 Three Rivers, CA 93271 Rebecca Wenk University of California Berkeley 137 Mulford Hall Berkeley, CA 94720-3114 Sylvia Haultain Sequoia and Kings Canyon National Parks 47050 Generals Highway Three Rivers, CA 93271 June 2013 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. -

Literature Cited
Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volume 9, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991), Bridson (2004), and Bridson and D. W. Brown (http://fmhibd.library.cmu.edu/fmi/iwp/cgi?-db=BPH_Online&-loadframes). When those forms are abbreviated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and Stafleu et al. (1992–2009). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix “a”; thus, the sequence of explicit suffixes begins with “b”. -

Abies Bracteata Revised 2011 1 Abies Bracteata (D. Don) Poit
Lead Forest: Los Padres National Forest Forest Service Endemic: No Abies bracteata (D. Don) Poit. (bristlecone fir) Known Potential Synonym: Abies venusta (Douglas ex Hook.) K. Koch; Pinus bracteata D. Don; Pinus venusta Douglas ex Hook (Tropicos 2011). Table 1. Legal or Protection Status (CNDDB 2011, CNPS 2011, and Other Sources). Federal Listing Status; State Heritage Rank California Rare Other Lists Listing Status Plant Rank None; None G2/S2.3 1B.3 USFS Sensitive Plant description: Abies bracteata (Pinaceae) (Fig. 1) is a perennial monoecious plant with trunks longer than 55 m and less than 1.3 m wide. The branches are more-or-less drooping, and the bark is thin. The twigs are glabrous, and the buds are 1-2.5 cm long, sharp-pointed, and non- resinous. The leaves are less than 6 cm long, are dark green, faintly grooved on their upper surfaces, and have tips that are sharply spiny. Seed cones are less than 9 cm long with stalks that are under15 mm long. The cones have bracts that are spreading, exserted, and that are 1.5–4.5 cm long with a slender spine at the apex. Taxonomy: Abies bracteata is a fir species and a member of the pine family (Pinaceae). Out of the fir species growing in North America (Griffin and Critchfield 1976), Abies bracteata has the smallest range and is the least abundant. Identification: Many features of A. bracteata can be used to distinguish this species from other conifers, including the sharp-tipped needles, thin bark, club-shaped crown, non-resinous buds, and exserted spine tipped bracts (Gymnosperms Database 2010).