DNA Content, Karyotypes, and Chromosomal Location of 18S-5.8S-28S Ribosomal Loci in Some Species of Bivalve Molluscs from the Pacific Canadian Coast
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CROSS-SHELF TRANSPORT of PLANKTONIC LARVAE of INNER SHELF BENTHIC INVERTEBRATES LAURA ANN BRINK a THESIS Presented to the Depart
CROSS-SHELF TRANSPORT OF PLANKTONIC LARVAE OF INNER SHELF BENTHIC INVERTEBRATES by LAURA ANN BRINK I, A THESIS Presented to the Department ofBiology and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Master ofScience March 1997 11 "Cross-Shelf Transport of Planktonic Larvae of Inner Shelf Benthic Invertebrates," a thesis prepared by Laura Ann Brink in partial fulfillment of the requirements for the Master of Science degree in the Department of Biology. This thesis has been approved and accepted by: the Examining Committee Date Committee in charge: Dr. Alan Shanks, Chair Dr. Steve Rumrill Dr. Lynda Shapiro Accepted by: Vice Provost and Dean of the Graduate School 111 An Abstract of the Thesis of Laura Ann Brink for the degree of Master of Science in the Department of Biology to be taken March 1997 Title: CROSS-SHELF TRANSPORT OF PLANKTONIC LARVAE OF INNER SHELF Approved: -l~P:::::::::~~~~'.1------- Ian Shanks There are two schools of thought on the affect of the physical environment in larval transport and dispersal. One is that larvae act as passive particles, being transported solely by physical means. The other idea is that through behavioral changes, larvae can alter their transport so as to improve their chances of successfully settling. Both cross-shelf and 23 hour vertical sampling of meroplanktonic larvae was conducted to investigate the hypothesis that larvae act as passive particles, being dispersed by the currents. The lengths ofbivalve larvae were also measured to determine if there were ontogenetic differences in the vertical distributions of these larvae. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1991 Elsevier B.V. The final published version of this manuscript is available at http://www.sciencedirect.com/science/journal/00448486 and may be cited as: Gustafson, R. G., Creswell, R. L., Jacobsen, T. R., & Vaughan, D. E. (1991). Larval biology and mariculture of the angelwing clam, Cyrtopleura costata. Aquaculture, 95(3-4), 257-279. doi:10.1016/0044-8486(91)90092-L Aquaculture, 95 (1991) 257-279 257 Elsevier Science Publishers B.V .. Amsterdam Larval biology and mariculture ofthe angelwing clam, Cyrtopleura costata KG. Gustafson"·l, R.L. Creswell", T.R. Jacobsen" and D.E. Vaughan" "Division olCoastal. Environmental and .iquacultural SCiCIICCS, Harbor Branch Oceanographic Institution, 5600 Old DixlC Highway, Fort Pierce, FL 34946, U,)',I "Department otMarinc and Coastal SCiCIICCS, Rutgers Shellfish Research l.aboratorv. New Jcrscv .tgnrultural Evperimcnt Station, Rutgers University, Port Norris, NJ 08349, USI (Accepted 7 November 1990) ABSTRACT Gustafson. R.G .. Creswell, R.L. Jacobsen. T.R. and Vaughan. D.E .. 1991. Larval biology and mari culture of the angclwing clam. Cvrtoplcura costata. Aquaculture, 95: 25 7~2 79. The deep-burrowing angclwing clam. Cvrtoplcura costata (Family Pholadidac ). occurs in shallow water from Massachusetts. USA. to Brazil and has been a commercially harvested food product in Cuba and Puerto Rico. This study examines its potential for commercial aquaculture development. The combined effects of salinity and temperature on survival and shell growth to metamorphosis of angclwing larvae were studied using a 5 X 5 factorial design: salinities ranged from 15 to 35%0 S. -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Marine Boring Bivalve Mollusks from Isla Margarita, Venezuela
ISSN 0738-9388 247 Volume: 49 THE FESTIVUS ISSUE 3 Marine boring bivalve mollusks from Isla Margarita, Venezuela Marcel Velásquez 1 1 Museum National d’Histoire Naturelle, Sorbonne Universites, 43 Rue Cuvier, F-75231 Paris, France; [email protected] Paul Valentich-Scott 2 2 Santa Barbara Museum of Natural History, Santa Barbara, California, 93105, USA; [email protected] Juan Carlos Capelo 3 3 Estación de Investigaciones Marinas de Margarita. Fundación La Salle de Ciencias Naturales. Apartado 144 Porlama,. Isla de Margarita, Venezuela. ABSTRACT Marine endolithic and wood-boring bivalve mollusks living in rocks, corals, wood, and shells were surveyed on the Caribbean coast of Venezuela at Isla Margarita between 2004 and 2008. These surveys were supplemented with boring mollusk data from malacological collections in Venezuelan museums. A total of 571 individuals, corresponding to 3 orders, 4 families, 15 genera, and 20 species were identified and analyzed. The species with the widest distribution were: Leiosolenus aristatus which was found in 14 of the 24 localities, followed by Leiosolenus bisulcatus and Choristodon robustus, found in eight and six localities, respectively. The remaining species had low densities in the region, being collected in only one to four of the localities sampled. The total number of species reported here represents 68% of the boring mollusks that have been documented in Venezuelan coastal waters. This study represents the first work focused exclusively on the examination of the cryptofaunal mollusks of Isla Margarita, Venezuela. KEY WORDS Shipworms, cryptofauna, Teredinidae, Pholadidae, Gastrochaenidae, Mytilidae, Petricolidae, Margarita Island, Isla Margarita Venezuela, boring bivalves, endolithic. INTRODUCTION The lithophagans (Mytilidae) are among the Bivalve mollusks from a range of families have more recognized boring mollusks. -

Physiological Adaptations and Feeding Mechanisms of the Invasive Purple Varnish Clam, Nuttallia Obscurata
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2013 Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata Leesa E. Sorber Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Biology Commons Recommended Citation Sorber, Leesa E., "Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata" (2013). WWU Graduate School Collection. 281. https://cedar.wwu.edu/wwuet/281 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. PHYSIOLOGICAL ADAPTATIONS AND FEEDING MECHANISMS OF THE INVASIVE PURPLE VARNISH CLAM, NUTTALLIA OBSCURATA by Leesa E. Sorber Accepted in Partial Completion of the Requirements for the Degree Master of Science Kathleen L. Kitto, Dean of the Graduate School ADVISORY COMMITTEE Chair, Dr. Deborah Donovan Dr. Benjamin Miner Dr. Jose Serrano-Moreno MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permission for the owner of any third party copyrighted material included in these files. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Marine Shells of the Western Coast of Flordia
wm :iii! mm ilili ! Sfixing cHdL J^oad .Sandivicl'i, j\{ai.i.ach.u±£.tti. icuxucm \^*^£ FRONTISPIECE Photo by Ruth Bernhard Spondylus americanus Hermann MARINE SHELLS f>4 OF THE WESTERN COAST OF FLORIDA By LOUISE M. PERRY AND JEANNE S. SCHWENGEL With Revisions and Additions to Louise M. Perry's Marine Shells of the Southwest Coast of Florida Illustrations by W. Hammersley Southwick, Axel A. Olsson, and Frank White March, 1955 PALEONTOLOGICAL RESEARCH INSTITUTION ITHACA, NEW YORK U. S. A. MARINE SHELLS OF THE SOUTHWEST COAST OF FLORIDA printed as Bulletins of American Paleontology, vol. 26, No. 95 First printing, 1940 Second printing, 1942 Copyright, 1955, by Paleontological Research Institution Library of Congress Catalog Card Number: 5-^-12005 Printed in the United States of America // is perhaps a more fortunate destiny to have a taste for collecting shells than to be born a millionaire. Robert Louis Stevenson imeters 50 lllllllllllllllllllllllllllll II II III nil 2 Inches CONTENTS Page Preface by reviser 7 Foreword by Wm. J. Clench 9 Introduction 11 Generalia 13 Collection and preparation of specimens 17 Systematic descriptions 24 Class Amphineura :. 24 Class Pelecypoda 27 Class Scaphopoda 97 Class Gasteropoda 101 Plates 199 Index 311 PREFACE BY THE REVISER It has been a privilege to revise Louise M. Perry's fine book on "Marine Shells of Southwest Florida", to include her studies on eggs and larvae of mollusks; and to add descriptions and illustra- tions of several newly discovered shells thus making it a more com- prehensive study of the molluscan life of western Florida. The work that I have done is only a small return to Dr. -
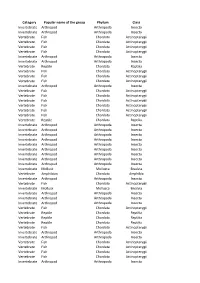
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Guide to Estuarine and Inshore Bivalves of Virginia
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1968 Guide to Estuarine and Inshore Bivalves of Virginia Donna DeMoranville Turgeon College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Marine Biology Commons, and the Oceanography Commons Recommended Citation Turgeon, Donna DeMoranville, "Guide to Estuarine and Inshore Bivalves of Virginia" (1968). Dissertations, Theses, and Masters Projects. Paper 1539617402. https://dx.doi.org/doi:10.25773/v5-yph4-y570 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. GUIDE TO ESTUARINE AND INSHORE BIVALVES OF VIRGINIA A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts LIBRARY o f the VIRGINIA INSTITUTE Of MARINE. SCIENCE. By Donna DeMoranville Turgeon 1968 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts jfitw-f. /JJ'/ 4/7/A.J Donna DeMoranville Turgeon Approved, August 1968 Marvin L. Wass, Ph.D. P °tj - D . dvnd.AJlLJ*^' Jay D. Andrews, Ph.D. 'VL d. John L. Wood, Ph.D. William J. Hargi Kenneth L. Webb, Ph.D. ACKNOWLEDGEMENTS The author wishes to express sincere gratitude to her major professor, Dr. -

The Mode of Life in the Genus Pholadomya As Inferred from the Fossil Record
geosciences Article The Mode of Life in the Genus Pholadomya as Inferred from the Fossil Record Przemysław Sztajner Institute of Marine and Environmental Sciences, University of Szczecin, Mickiewicza 16A, 70-383 Szczecin, Poland; [email protected] Received: 13 July 2020; Accepted: 21 September 2020; Published: 5 October 2020 Abstract: The paper is an attempt to reconstruct the mode of life of Pholadomya bivalves, very common in the fossil record, particularly that of the Jurassic. The only extant representative of the genus is extremely rare and very poorly known. Materials from the Polish Jurassic deposits (Bajocian–Kimmeridgian; Western Pomerania and Polish Jura) and literature data were used for the reconstruction. Specifically, observations on the anatomy, taphonomy, and diagenesis of the specimens examined as well as lithology of the deposits housing the specimens were used. Shell anatomy characteristics are known for their particular utility in mode of life reconstructions, although the extremely thin-shelled and coarsely sculpted bivalves, such as the Pholadomya examined, have not been studied so far. The reconstruction suggest a diversity of the mode of life, coincident with the morphological differences between the Pholadomya species. At least the adults of anteriorly flattened species are inferred to have lived extremely deeply buried in the sediment, and were hardly mobile. The smaller, more oval in shape, species were more mobile, and some of them are thought to have preferred life in shelters, should those be available. In addition, the function of the cruciform muscle, other than that considered so far, is suggested. Keywords: Pholadomya; Anomalodesmata; Bivalvia; life habit; deep burrowers; taphonomy; shell anatomy; cruciform muscle; Jurassic; Poland 1. -

Of Mitochondria: Investigating the Functioning, Maintenance and Evolutionary Relevance of a Naturally Heteroplasmic System
Université de Montréal Energy metabolism in species with Doubly Uniparental Inheritance (DUI) of mitochondria: investigating the functioning, maintenance and evolutionary relevance of a naturally heteroplasmic system Par Stefano Bettinazzi Département de sciences biologiques, Faculté des arts et des sciences Thèse présentée en vue de l’obtention du grade de Philosophiae Doctor (Ph.D.) en sciences biologiques June 2020 © Stefano Bettinazzi, 2020 A été évaluée par un jury composé des personnes suivantes Sandra Binning Président-rapporteur Sophie Breton Directeur de recherche Pierre Blier Codirecteur Annie Anger Membre du jury Ron Burton Examinateur externe RÉSUMÉ Les mitochondries et leur génome, l'ADN mitochondrial (ADNmt), sont généralement transmis uniquement par la mère aux fils et aux filles chez les métazoaires (transmission strictement maternelle, SMI). Une exception à la règle générale de la SMI se trouve dans environ 100 espèces de bivalves, qui se caractérise par une double transmission uniparentale (DUI) des mitochondries. Chez les espèces DUI, deux lignées d'ADNmt très divergentes et liées au sexe coexistent. Une lignée mitochondriale maternelle (type F), présente dans les ovocytes et les tissus somatiques des individus femelles et males, et une lignée paternelle (type M), présente dans les spermatozoïdes. Dans les tissus somatiques mâles, les deux lignées coexistent parfois, une condition appelée hétéroplasmie. En sachant que les variations génétiques dans l’ADNmt peuvent avoir un impact sur les fonctions mitochondriales, et en donnant l'association stricte des ADNmt de type M et F avec différents gamètes, il est imaginable que la forte divergence entre les deux lignées DUI puisse entraîner des adaptations bioénergétiques avec répercussion sur la reproduction. -

Invertebrate ID Guide
11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-48/htm/doc.html Polychaetes http://web.vims.edu/bio/benthic/polychaete.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-34/htm/doc.html Cephalopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-44/htm/doc.html Amphipods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-67/htm/doc.html Molluscs http://www.oceanica.cofc.edu/shellguide/ http://www.jaxshells.org/slife4.htm Bivalves http://www.jaxshells.org/atlanticb.htm Gastropods http://www.jaxshells.org/atlantic.htm Crustaceans http://www.jaxshells.org/slifex26.htm Echinoderms http://www.jaxshells.org/eich26.htm 2 PROTOZOA (FORAMINIFERA) ................................................................................................................................ 4 PORIFERA (SPONGES) ............................................................................................................................................... 4 CNIDARIA (JELLYFISHES, HYDROIDS, SEA ANEMONES) ............................................................................... 4 CTENOPHORA (COMB JELLIES)............................................................................................................................