Table of Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optimization of Electrolysis Parameters for Green Sanitation Chemicals Production Using Response Surface Methodology
processes Article Optimization of Electrolysis Parameters for Green Sanitation Chemicals Production Using Response Surface Methodology Nurul Izzah Khalid 1 , Nurul Shaqirah Sulaiman 1 , Norashikin Ab Aziz 1,2,* , Farah Saleena Taip 1, Shafreeza Sobri 3 and Nor-Khaizura Mahmud Ab Rashid 4 1 Department of Process and Food Engineering, Faculty of Engineering, Universiti Putra Malaysia, UPM Serdang 43400, Selangor, Malaysia; [email protected] (N.I.K.); [email protected] (N.S.S.); [email protected] (F.S.T.) 2 Halal Products Research Institute, University Putra Malaysia, UPM Serdang 43300, Selangor, Malaysia 3 Department of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia, UPM Serdang 43400, Selangor, Malaysia; [email protected] 4 Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, UPM Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected]; Tel.: +603-9769-4302 Received: 22 May 2020; Accepted: 10 June 2020; Published: 6 July 2020 Abstract: Electrolyzed water (EW) shows great potential as a green and economical sanitation solution for the food industry. However, only limited studies have investigated the optimum electrolysis parameters and the bactericidal effect of acidic electrolyzed water (AcEW) and alkaline electrolyzed water (AlEW). Here, the Box–Behnken experimental design was used to identify the optimum parameters. The tests were conducted with different types of electrodes, electrical voltages, electrolysis times, and NaCl concentrations. There were no obvious differences observed in the physico-chemical properties of EW when different electrodes were used. However, stainless steel was chosen as it meets most of the selection criteria. -

Full Agreement
CONSULTATIONS AND WORKSHOPS Benefits and Risks of the Use of Chlorine-containing Disinfectants in Food Production and Food Processing Report of a Joint FAO/WHO Expert Meeting Ann Arbor, MI, USA, 27–30 May 2008 Use of Chlorine-containing Disinfectants in Food Production and Food Processing WHO Library Cataloguing-in-Publication Data Benefits and risks of the use of chlorine-containing disinfectants in food production and food processing: report of a joint FAO/WHO expert meeting, Ann Arbor, MI, USA, 27–30 May 2008. 1.Food contamination. 2.Chlorine - toxicity. 3.Disinfectants - toxicity. 4.Disinfection - utilization. 5.Food hygiene - standards. 6.Risk assessment. I.World Health Organization. II.Food and Agriculture Organization of the United Nations. ISBN 978 92 4 159894 1 (NLM classification: WA 701) ISBN 978 92 5 106476 4 © FAO and WHO 2009 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) or of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Menu PDF Download
“The Perfect FISH TACO” PŪ-PŪ (Appetizers) Tacos KAMA'AINA FAVORITES COCONUT SHRIMP .........................$8.99 FISH TACOS ............................$10.99 poke bowl* ............................. $12.99 Six large shrimp served with house made Thai chili pineapple sauce Two tacos per order made with 17 ingredients; including grilled Hawaiian favorite featuring raw Ahi Poke, pickled cucumber, ginger, and Ono and Mahi Mahi, white corn tortilla, organic tomato salsa, red onion; with radish, mangos, edamame, and avocado; over brown . CALAMARI $8.99 cheese, house made coleslaw and mango salsa rice and mixed greens. Available as original or spicy style Lightly battered steaks cut into strips and served with house made cocktail sauce SHRIMP TACOS .......................... $11.99 Teriyaki Chicken Bowl . $11.99 SHRIMP Panko Crusted . $8.99 Two tacos per order made with 17 ingredients Hawaiian Teriyaki Chicken served with poke bowl fxings Six large shrimp served with our house made cocktail sauce STEAK, CHICKEN, OR VEGGIE TACOS .. $10.99 MACADAMIA NUT MAHI MAHI . $15.99 GRILLED SHRIMP SKEWERS . $9.99 Two tacos per order made with 17 ingredients Coconut mango sauce, brown rice and famous coconut milk Eight grilled shrimp served with our house made cocktail sauce coleslaw or substitute steamed vegetables AHI POKE* . $11.99 TACO MOUNTAIN .....................$11.99 Raw Hawaiian classic available as original or spicy style, Choice of fsh, steak, chicken, shrimp (+$1.00) or veggie patty. served with house made taro chips Served with all the taco fxings over brown rice Pasta SEAFOOD PASTA . .$16.99 Alfredo cream sauce with capers or marinara sauce with Mahi Mahi, Ono, and shrimp. Slice of garlic bread. -

Electrolysed Oxidising Water
Electrolysed Oxidising Water TECHNOLOGY SUMMARY Status An emerging technology Location Pre- or post-slaughter Intervention type Surface treatment of hide or warm carcase Treatment time 15-30 seconds Regulations Approved in US and considered safe in Japan and Australia, but awaiting full approval. Not approved for the EU for carcass washing, but approved for treatment of drinking water. Approved for water treatment and equipment sanitising in New Zealand Effectiveness 1.5-3 log reduction Likely cost Packaged units range from AU$20,000 to AU$75,000 Value for money Substantially cheaper than other treatments (e.g., organic acid chemicals are more expensive than salt). However, even though salt would be cheaper, the equipment costs would be similar to any surface treatment equipment Plant or process Minimal change; can use existing plumbing. Space may be changes required for a spray cabinet treatment area if none existing Environmental impact Environmentally friendly solution, may in fact improve quality of effluent Energy would be required to produce the current OH&S Solution is harmless, unit is fully enclosed so little safety issue from electric current generated as part of process Advantages Salt is the only chemical used Little corrosion of stainless steel and carbon steel when using EO water Disadvantages or Need a spray cabinet to apply treatment to carcases limitations Depending on the pressure of application, there may be issues with water penetration into the fat surfaces Page 1 of 4 Disclaimer Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. -

A Review of Guidance on Fish Consumption in Pregnancy: Is It Fit for Purpose? Public Health Nutrition
Taylor, C. , Emmett, P., Emond, A., & Golding, J. (2018). A review of guidance on fish consumption in pregnancy: Is it fit for purpose? Public Health Nutrition. https://doi.org/10.1017/S1368980018000599 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1017/S1368980018000599 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Cambridge University Press at https://www.cambridge.org/core/journals/public-health-nutrition/article/review-of-guidance-on- fish-consumption-in-pregnancy-is-it-fit-for-purpose/BC3BB20A2D848F5CF5AED90C86413F85 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Public Health Nutrition: page 1 of 11 doi:10.1017/S1368980018000599 Review Article A review of guidance on fish consumption in pregnancy: is it fit for purpose? Caroline M Taylor*, Pauline M Emmett, Alan M Emond and Jean Golding Centre for Child and Adolescent Health, Population Health Sciences, Bristol Medical School, University of Bristol, Oakfield House, Oakfield Grove, Bristol BS8 2BN, UK Submitted 17 November 2017: Final revision received 14 February 2018: Accepted 14 February 2018 Abstract Objective: Public health messages to reduce Hg exposure for pregnant women have focused exclusively on advice on fish consumption to limit Hg exposure, with little account being taken of the positive contribution of fish to nutritional quality. -
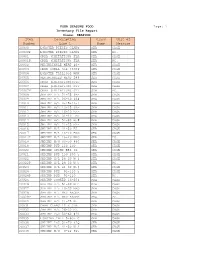
Page: 1 Item Description Class Unit Of
FOUR SEASONS FOOD Page: 1 Inventory File Report Class: SEAFOOD Item Description Class Unit of Number Line 1 Name Measure 20000 LOBSTER PIECES CLAW& SEA CASE 20000P LOBSTER PIECES CLAW& SEA PC. 20001 CRAB (IMITATION) FLA SEA CASE 20001P CRAB (IMITATION) FLA SEA PC. 20002 PERIWRINKLE MEAT 40- SEA CASE 20003 CRAB SHELL 3oz (300P SEA CASE 20004 LOBSTER TAIL10oz WAR SEA CASE 20005 PERIWINKLES MEAT 24# SEA CASE 20006 CRAB (IMITATION)STIC SEA CASE 20007 CRAB (IMITATION) STI SEA CASE 20007P CRAB (IMITATION) STI SEA PC. 20008 SHRIMP H/L 21-25 (B- SEA CASE 20009 SHRIMP H/L 26-30 40# SEA CASE 20010 SHRIMP H/L U-15&13-1 SEA CASE 20011 SHRIMP H/L 13-15 (B- SEA CASE 20012 SHRIMP H/L 16-20 EZ- SEA CASE 20013 SHRIMP H/L 31-40 EZ SEA CASE 20014 SHRIMP H/L 51-60 W.# SEA CASE 20015 SHRIMP H/L 21-25 EZ- SEA CASE 20016 SHRIMP H/L 41-50 EZ SEA CASE 20017 SHRIMP H/L 16-20 BRO SEA CASE 20017P SHRIMP H/L 16-20 BRO SEA PC. 20018 SHRIMP H/O 40-50 #40 SEA CASE 20019 SHRIMP PUD 110-130 SEA CASE 20020 SHRIMP SUSHI EBI 4L SEA CASE 20021 SHRIMP PUD 150-200 5 SEA CASE 20022 SHRIMP H/L 26-30 W 3 SEA CASE 20022P SHRIMP H/L 26-30 W 5 SEA PC. 20023 SHRIMP H/L 41-50 W.# SEA CASE 20024 SHRIMP PUD 91-110 5 SEA CASE 20024P SHRIMP PUD 91-110 SEA PC. -

UNITED STATES PATENT OFFICE 1989,383 METHOD of TREATING SEAFOOD and the PRODUCT RESULT ING THEREFRORM Charles H
Patented Jan. 29, 1935 1989,383 UNITED STATES PATENT OFFICE 1989,383 METHOD OF TREATING SEAFOOD AND THE PRODUCT RESULT ING THEREFRORM Charles H. Schuh, Glendale, Long Island, N. Y., assignor to Sturmack Company, Inc., Brooklyn, N. Y., a corporation of Delaware No Drawing. Applicatin August 2, 1931, Serial No. 559,775. Renewed June 16, 1934 9 Claims. (C. 99-11) The present invention relates to a method of like product. The jelly-like product is packed treating seafood and to the product resulting into a Suitable mold Without the loss of any drip threfrom and more particularly to a method of page. The fish in the mold is baked and a baked treating seafood to produce a baked and Smoked fish steak is produced without the loss of juices. 5 Steak and to a baked and smoked seafood steak The baked steak of seafood is Smoked under the produced by said method. - . influence of heat. The Smoked steak can be han It is well known that when fish is boned and dled and sold as a unit, can be cut or sliced, and converted to the fillet condition, a product is ob can be eaten directly as a foodstuff. For a better tained which has a watery consistency and which understanding of the invention, by those skilled in 10 tends to lose a portion of its juices as soon as it is the art, and for illustrative purposes, the following: COmpressed Or processed in some manner. The art specific examples are given. 10 has been in want of a steak or shaped unit of sea food which is edible and ready for consumption Eacample No. -

Expert Group for Technical Advice on Organic Production EGTOP Final
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR AGRICULTURE AND RURAL DEVELOPMENT Directorate B. Multilateral relations, quality policy B.4. Organics Estratto delle pag 42,43 e 44: Expert Group for Technical Advice on Organic Production EGTOP Final Report on Cleaning and Disinfection The EGTOP adopted this technical advice at the 12th plenary meeting of 14 – 15 • calcium magnesium carbonate (dolomite) [no restriction proposed] • cleaning products which do not contain active disinfectant substances • Ecolabelled (at least to EU standard) cleaning products 1.2 List of substances for cleaning and disinfection, which may be used for limited purposes indicated here (only if other substances listed in chapter 2.1 of this Annex cannot be used): • iodophors (only in the presence of eggs) • cleaning products which are not ecolabelled, only if there are no suitable ecolabelled products 2. Products for use in aquaculture, only in the absence of animals 2.1 Basic list of substances for cleaning and disinfection, which may be used for all purposes authorised under general legislation: • ethanol, propan-2-ol (alcohols) • sodium hydroxide (caustic soda), calcium hydroxide (slaked lime) • calcium oxide (quicklime) • sodium hypochlorite (bleach), calcium hypochlorite, mixtures of potassium peroxomonosulphate and sodium chloride producing hypochlorous acid • ozone • cleaning products which do not contain active disinfectant substances • Ecolabelled (at least to EU standard) cleaning products 2.2 List of substances for cleaning and disinfection, which may be -

Effects of Treatment with Electrolyzed Oxidizing Water on Postharvest
agriculture Article Effects of Treatment with Electrolyzed Oxidizing Water on Postharvest Diseases of Avocado Md Kamrul Hassan 1,2 and Elizabeth Dann 1,* 1 Centre for Horticultural Science, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, 2CW Ecosciences Precinct, Dutton Park, Brisbane QLD 4102, Australia; [email protected] 2 Department of Horticulture, Faculty of Agriculture, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh * Correspondence: [email protected] Received: 14 October 2019; Accepted: 29 October 2019; Published: 11 November 2019 Abstract: The present study comprised five trials to investigate the efficacy of postharvest treatment with electrolyzed oxidizing (EO) water on postharvest disease development in avocado. Mature (dry matter 24–34%), hard green fruit cv. Hass (four trials), and cv. Wurtz (one trial) from orchards receiving minimal fungicide sprays were sourced, and subsequently dipped for 30 s in treatment solutions. Fruit were ripened at 23 ◦C and 65% relative humidity to encourage postharvest disease development, and assessed when ripe for anthracnose and stem end rot (SER), arising from natural field infections and/or the size of lesions resulting from post-treatment inoculation with Colletotrichum siamense. In the case of natural infection, EO water treatment reduced severity of SER disease by 30–75% compared with water treated control fruit in all four trials where it was assessed. Reduction in severity of SER after Graduate A+ fungicide or hypochlorite (NaOCl) bleach treatment ranged from 60–88% or 25–50%, respectively, compared with water controls. Under extremely high anthracnose disease pressure, 20% v/v EO water, NaOCl, as well as Graduate A+ fungicide treatments were mostly ineffective. -

Fish & Seafood
On-Site Generated Electrolyzed Water FISH & SEAFOOD SanClean-G240, SanClean-G1200 ECA Generators Safe. Natural. Effective. +44 161 820 8441| [email protected] | www.eaquasys.com About Us Eaquasys manufactures and distributes compact systems for on-site generation of electrolysed water, a powerful disinfectant with applications along the entire food chain. What is Electrolysed Water Electrolysed water is an all-natural, non-toxic, non-hazardous, and pH neutral anti-microbial solution. Electrolysed water is 100 times more effective than chlorine bleach as a disinfectant yet so safe that it can be ingested without any harm. The active molecule in electrolysed water is hypochlorous acid (HOCl), which is generated by the electrolysis of two natural ingredients, water and salt. HOCl is a free chlorine molecule that is safe, non-toxic, and non-irritant. It is also a natural molecule in our blood. It is produced by our white blood cells to fight infection from bacteria and viruses. Benefits as a disinfectant • Effective against all common foodborne pathogens including E. coli, Salmonella, and Listeria. • Effective against parasites and viruses • Effective against Vibrio parahaemolyticus • Effective against Legionella contamination • Dislodges biofilm in plumbing and prevents regrowth • Neutral pH and compatible with water networks • Does not cause corrosion of stainless steel • Biodegradable and safe for the environment • Useful for producing sanitised ice for seafood • Eliminates the need for harsh chemicals and additives The pH neutral electrolysed -

Hydro33 Is a Hypochlorous Acid (HOCI) Solution Which Also Known As Electrolysed Water Containing Monovalent Chlorine That Acts As an Oxidizing Or Reducing Agent
MaHTAS COVID-19 RAPID EVIDENCE UPDATES HYPOCHLORUS ACID FOR INDOOR AND OUTDOOR DISINFECTANT Based on available evidence up to 20 May 2020 INTRODUCTION Hydro33 is a hypochlorous acid (HOCI) solution which also known as electrolysed water containing monovalent chlorine that acts as an oxidizing or reducing agent. It is produced by the electrolysis of water-sodium chloride solution. The solution is commonly used in wound care in health setting. Hypochlorous acid is known to be detrimental to bacterial cells resulting from destructive oxidation of membrane bound complexes involved in ATP generation and maintenance of cellular electrical charge.1 The United States Food Drug Administration (US FDA) approved usage of hypochlorous acid in food safety, food produce, seafood, meat and poultry sanitation.2 Hypochlorous acid containing solution has been listed by the United States Environmental Protection Agency (EPA) for disinfectants for use against SARS-CoV-2 however, Hydro33 itself is not registered with the agency.3 This rapid literature review was conducted to determine the efficacy, safety and cost effectiveness of Hydro33 as surface indoor and outdoor disinfectant for COVID-19 or coronavirus infection. EVIDENCE ON EFFECTIVENESS AND SAFETY The systematic search for new evidence from the scientific databases such as Medline, EBM Reviews, EMBASE via OVID, PubMed and from the general search engines [Google Scholar and US Environmental Protection Agency (US EPA)], and yielded two articles related to effectiveness of hypochlorous acid as surface disinfectant indoor or outdoor. However, there was no study retrieved on the safety and cost-effectiveness. Park et. al (2007) conducted an evaluation to determine effectiveness of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of Human Norovirus. -

Chemical Composition and Fatty Acid Profile of Lipids in Carp (Cyprinus Carpio L.) Meat As Affected by Cooking Methods
73 Bulgarian Journal of Agricultural Science, 24 (Suppl. 2) 2018, Agricultural Academy CHEMICAL COMPOSITION AND FATTY ACID PROFILE OF LIPIDS IN CARP (CYPRINUS CARPIO L.) MEAT AS AFFECTED BY COOKING METHODS ANGELINA IVANOVA1*; MARIA ANGELOVA-ROMOVA2; ZHANA PETKOVA2; GINKA ANTOVA2; TANIA HUBENOVA1 1Agricultural Academy, Institute of Fisheries and Aquaculture, 248 V. Levski Str., 4003 Plovdiv, Bulgaria 2University of Plovdiv “Paisii Hilendarski”, Department of Chemical Technology, 24 Tzar Asen Str., 4000 Plovdiv, Bulgaria Abstract Ivanova A. S., M. J. Angelova-Romova, Z. Y. Petkova, G. A. Antova and T. A. Hubenova, 2018. Chemical com- position and fatty acid profile of lipids in carp Cyprinus( carpio L.) meat as affected by cooking methods. Bulg. J. Agric. Sci.,24 (Suppl. 2): 73-80 The aim of the present study is to analyze the chemical composition of the meat and fatty acid profile of carp lipids (Cy- prinus carpio L.) before and after heat treatment (baking and frying). The water content (weight method), proteins (Kjeldahl procedure), fat (arbitrage method), ash (weight method) and fatty acid composition (GC) are determined for carp from aqua- culture – pond – and cage reared and for free-living carp from Zhrebchevo dam lake. It has been established that the level of water in the baked and fried carp decreases as a result of the heat treatment. Increases in protein, fat and ash content in heat treated meat samples compared to fresh carp meat are reported. The fatty acid profile of baked and fried carp showed a slight decrease in the level of saturated and monounsaturated fatty acids. An increase in the percentage of polyunsaturated fatty acids as a result of heat treatment is reported.