The Science and Engineering of Materials, 4Th Ed Donald R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ferritic Nitrocarburizing Gears to Increase Wear Resisitance And
Ferritic Nitrocarburizing Gears tOI Increase Wear Resistance and Reduce Distortion Loren ,JI, Epler uaHtygear manufacturing depends on controlled toler- asa gaseous territic nitrocarl:mrizillg process and patented by ances and geometry. As a re ult, ferritic nillocarburizing Lucas Industries in 1961. Lucas demonstrated that they could ha become the heat treat process of choice for many produce surface layers identical to those produced in salt bath gear manufacturers. The primary reason for this are: processes using an endothermic, ammonia-based atmosphere. The process is performed at low temperatures, i.e. less than The process was classified as a "thermochemical susfece critical treatment" that involved !he diffusion of both nitrogenand car- 2. The quench methods increase fatigue strength by up to, L25% bon into the surface of a metal at a temperature below the au tell- without distorting. Ferritlc nitrocarboriziag is used in place ite transforrnation temperature. The process would yield .3 single of carburizing and hardening, carbonitriding, nltriding or in phase epsilon layer with an atomic weight of Fep3' The ingle conjunction wi.th conventional and induction hardening. phase layer makes !:heproduct much more wear resistant than gas 3. h establishes gradient base haronesses, i.e, eliminates egg- or ionnitriding, according to Dawc and Trantner 0). shell effect on TiN, TiAlN,. ere. etc. [II 1982, lronbeund HeatTreat developed Ni.tmwear® using In addition, the process can also be applied to hobs, broaches, similar atmo pheres in a fluidized bed medium. Subsequently. drills and other cutting tools. Jack Ross, owner and founder of lronbound,. patented and HisUJry, Fenitic nitrocarburizing was first established in licensed the process to Dynamic Metal Treating. -

Carbonitriding and Hard Shot Peening for High-Strength Gears Yoshihisa Miwa, Masayuki Suzawa, Yukio Arimi, Yoshihiko Kojima, and Katsunori Nishimura Mazda Motor Corp
Carbonitriding and Hard Shot Peening for High-Strength Gears Yoshihisa Miwa, Masayuki Suzawa, Yukio Arimi, Yoshihiko Kojima, and Katsunori Nishimura Mazda Motor Corp. ABSTRACT CUE HARONESS HARONESS CUE OEtrM A new process for manufacturing high- OISTRIBUTION --E CORE HARONESS strength gears has been developed to meet the requirement of automobile transmission minia- r turization. The points of the process are to GRAIN SIZE increase the shot peening intensity and to MICROSTRUCTURE perform optimal control of the initial (before FATIGUE CARBIOES shot peening) microstructure by heat treatment STREMGTH MON-MARTEMSITIC corresponding with the peening intensity in OEFEtTS -r SURFACEUOU-METALLIC LAYER order to obtain higher residual compressive IuCLUSlONS stress. INTERGRANULAR i TOUGHMESS The new process, named Carbonitriding and Hard Shot Peening in Mazda, brings a much RESlOUAL COYIRESIVE STRESS higher fatigue strength than the one obtained by the conventional carburizing and shot peening process. Fig. 1 METALLURGICAL FACTORS AFFECTING ON THE FATIGUE STRENGTH OF GEARS 1. Introduction A higher drivability, stability in therefore, it is necessary to set a suitable driving, and fuel efficiency are required for i~ardnessdistribution by heat treatment using automobiles and the new systems to meet these clean material. However, in order to augment requirements have been developed. This can the fatigue strength furthermore, it is nec- be seen, for example, in multi-valve system, essary to make use of residual compressive super charging in engines, 4 WD or 4 WS, etc. stress more positively. In addition, the height of engine hood has For enlargement of the residual compressive been lowered in body designing for reducing stress, it is most effective to increase the air resistance. -

Carburizing & Carbonitriding
CARBURIZING & CARBONITRIDING A TRADITIONAL SURFACE HARDENING TREATMENT WITH MODERN PROCESS CONTROLS WHAT IS IT? HOW IS IT DONE? General Description of Carburizing & Carbonitriding Equipment & Process Carburizing is a process of controlled diffusion of carbon into Solid, molten salt and gaseous carbon-carrying medium may the surface of a component, followed by quenching and be employed, however, the first two are now rarely used. tempering, with the objective of increasing the component’s Nitrex offers gas carburizing in computer controlled integral surface hardness. The process is generally applicable to low quench and pit gas carburizing furnaces. A full range of case carbon and low alloy steels. There are two carburizing depths if feasible with an economically derived limit of process types available commercially – vacuum carburizing approximately 0.250” (6.4 mm). In addition, Nitrex offers and conventional carburizing. The former is described in a vacuum carburizing which is described in a separate separate brochure, and conventional carburizing is company publication. discussed here. The sequence of operations is as follows : In this thermal process ferrous alloys are heated to above • parts are appropriately fixtured, loaded into the furnace, their transformation temperature and exposed to carbon rich and the process is initiated atmosphere. Processing temperatures in conventional • carburizing typically are in the 1450°F - 1900°F (790°C - computers and/or process controllers are programmed to 1040°C) range. The diffusion of carbon into the part and the conduct the process automatically, subsequent quench leads to a part with a hard, wear • at the end of the process the load is either direct resistant surface and a tough, shock resistant core. -

Distortion in Case Carburized Components- the Steelmakers View
Heat Treating: Proceedings of the 18th Conference Copyright © 1999 ASM International® Ronald A. Wallis, Harry W. Walton, editors, p 5-11 All rights reserved. DOI: 10.1361/cp1998ht005 www.asminternational.org Distortion in Case Carburized Components The Steel makers View M. Cristinacce British Steel Engineering Steels Rotherham, United Kingdom 1. Abstract NVH (noise, vibration and harshness) is becoming of increasing importance in the automotive industry. The control Distortion of components during heat treatment has a of distortion to give correct mating of rotating components significant effect upon final component costs. The factors such as gears and shafts has a major influence upon NVH which influence distortion behaviour occur during the performance. machining and heat treatment processes and are therefore Most of the examples given in this paper are automotive outside the control of the steelmaker. One important factor transmission components which are carburised. which is under the control of the steelmaker is hardenability A wide variety of factors influence distortion behaviour Consistent hardenability performance can have a significant and can be broadly summarised as follows: effect in reducing the variability in distortion. In a number of Component shape. instances it has been shown that the macrostructure and Steel type. as-cast shape of the steel can also influence distortion. Other Microstructure and residual stresses prior to heat downstream processing effects such as forging may also be treatment. influential in these circumstances. Reheating and carburising conditions. This paper gives examples of some of the experiences of Stacking and support in furnace. British Steel Engineering Steels with customers and end users, Quenching - Medium, temperature, flow, jigging, etc. -

Carburizing & Carbonitriding Control Solutions For
CARBURIZING & CARBONITRIDING CONTROL SOLUTIONS FOR BATCH FURNACES RETROFITS & NEW FURNACES CARBURIZING & CARBONITRIDING CONTROL SOLUTIONS OTHER CONTROL SOLUTIONS STANDARD EQUIPMENT • Protherm 455, 500, 600 or 700 controller (see separate brochures for more details) • Hi-limit temperature controllers for furnace & oil bath • Steel plate (mountable) or cabinet (enclosure) • DIN mounted hardware • Pre-wired with terminal blocks & isolation relays • Electrical drawing / user manual • Smart transmitter (allows probe care) Annealing MAIN FEATURES • Precise atmosphere control (carbon potential control) • Furnace temperature control • Quench (oil) temperature control • Alarm notification and processing • Password protected interface • Real time and historical paperless chart recorder displaying process variables • Recipes and templates can be created and modified Induction OPTIONAL FEATURES • Online carbon & nitrogen diffusion modulefor more precise control. Includes: target control / surface carbon control / soot carbon control / auto boost / auto diffusion / calculates carbon profile & hardness curve • ß-control module for more precise control & savings. Includes: online carbon diffusion module plus shortens process time and reduces energy, gas consumption and exhaust • Dual probe reliability function (with additional smart Nitriding transmitter) • Additional I/O cards: 3-gas I/R, Cascade heating control INTEGRATION / CONNECTION • Integrated web server • CANopen / DeviceNet interfaces • RS485/422 4 wire Modbus / J-bus • RJ45 Ethernet LAN interface for configuration • Seamless integration with SCADA • Modbus / TCP interface Vacuum • Optional Profibus slave or master USA CANADA CHINA FRANCE GERMANY POLAND +1 414 462 8200 +1 514 335 7191 +86 21 3463 0376 +33 3 81 48 37 37 +49 7161 94888 0 +48 32 296 66 00 [email protected] [email protected] [email protected] [email protected] [email protected] [email protected] Copyright © United Process Controls (Broc701rev7). -

An Introduction to Nitriding
01_Nitriding.qxd 9/30/03 9:58 AM Page 1 © 2003 ASM International. All Rights Reserved. www.asminternational.org Practical Nitriding and Ferritic Nitrocarburizing (#06950G) CHAPTER 1 An Introduction to Nitriding THE NITRIDING PROCESS, first developed in the early 1900s, con- tinues to play an important role in many industrial applications. Along with the derivative nitrocarburizing process, nitriding often is used in the manufacture of aircraft, bearings, automotive components, textile machin- ery, and turbine generation systems. Though wrapped in a bit of “alchemi- cal mystery,” it remains the simplest of the case hardening techniques. The secret of the nitriding process is that it does not require a phase change from ferrite to austenite, nor does it require a further change from austenite to martensite. In other words, the steel remains in the ferrite phase (or cementite, depending on alloy composition) during the complete proce- dure. This means that the molecular structure of the ferrite (body-centered cubic, or bcc, lattice) does not change its configuration or grow into the face-centered cubic (fcc) lattice characteristic of austenite, as occurs in more conventional methods such as carburizing. Furthermore, because only free cooling takes place, rather than rapid cooling or quenching, no subsequent transformation from austenite to martensite occurs. Again, there is no molecular size change and, more importantly, no dimensional change, only slight growth due to the volumetric change of the steel sur- face caused by the nitrogen diffusion. What can (and does) produce distor- tion are the induced surface stresses being released by the heat of the process, causing movement in the form of twisting and bending. -
Materialspec Materials
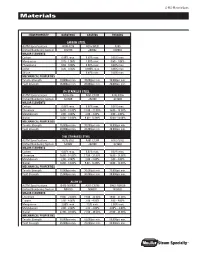
S-MS-MaterialSpec Materials Item/ProPerty Barstock castIng ForgIng carBon steel ASTM Specifications A108-1215 A216-WCB A105 Unified Numbering System G12150 J03002 K03504 maJor elements Carbon 0.09% max. 0.30% max. 0.35% max. Manganese 0.75 - 1.05% 1.00% max. 0.60 - 1.05% Phosphorus 0.04 - 0.09% 0.04% max. 0.04% max. Sulfur 0.26 - 0.35% 0.045% max. 0.05% max. Silicon - 0.60% max. 0.35% max. mecHanIcal ProPertIes Tensile Strength 70,000psi min. 70,000psi min. 70,000psi min. Yield Strength 36,000psi min. 36,000psi min. 36,000psi min. 316 staInless steel ASTM Specifications A276-316 A351-CF8M A182-F316 Unified Numbering System S31600 J92900 S31600 maJor elements Carbon 0.08% max. 0.08% max. 0.08% max. Chromium 16.00 - 18.00% 18.00 - 21.00% 16.00 - 18.00% Molybdenum 2.00 - 3.00% 2.00 - 3.00% 2.00 - 3.00% Nickel 10.00 - 14.00% 9.00 - 13.00% 10.00 -14.00% mecHanIcal ProPertIes Tensile Strength 75,000psi min. 70,000psi min. 75,000psi min. Yield Strength 30,000psi min. 30,000psi min. 30,000psi min. 316l staInless steel ASTM Specifications A276-316L A351-CF3M A182-F316L Unified Numbering System S31603 J92800 S31603 maJor elements Carbon 0.03% max. 0.03% max. 0.03% max. Chromium 16.00 - 18.00% 17.00 - 21.00% 16.00 - 18.00% Molybdenum 2.00 - 3.00% 2.00 - 3.00% 2.00 - 3.00% Nickel 10.00 - 14.00% 9.00 - 13.00% 10.00 - 15.00% mecHanIcal ProPertIes Tensile Strength 70,000psi min. -

Furnace Atmospheres No 1: Gas Carburising and Carbonitriding
→ Expert edition Furnace atmospheres no. 1. Gas carburising and carbonitriding. 02 Gas carburising and carbonitriding Preface. This expert edition is part of a series on process application technology and know-how available from Linde Gas. It describes findings in development and research as well as extensive process knowledge gained through numerous customer installations around the world. The focus is on the use and control of furnace atmospheres; however a brief introduction is also provided for each process. 1. Gas carburising and carbonitriding 2. Neutral hardening and annealing 3. Gas nitriding and nitrocarburising 4. Brazing of metals 5. Low pressure carburising and high pressure gas quenching 6. Sintering of steels Gas carburising and carbonitriding 03 Passion for innovation. Linde Gas Research Centre Unterschleissheim, Germany. With R&D centres in Europe, North America and China, Linde Gas is More Information? Contact Us! leading the way in the development of state-of-the-art application Linde AG, Gases Division, technologies. In these R&D centres, Linde's much valued experts Carl-von-Linde-Strasse 25, 85716 Unterschleissheim, Germany are working closely together with great access to a broad spectrum of technology platforms in order to provide the next generation of [email protected], www.heattreatment.linde.com, atmosphere supply and control functionality for furnaces in heat www.linde.com, www.linde-gas.com treatment processes. As Linde is a trusted partner to many companies in the heat treatment industry, our research and development goals and activities are inspired by market and customer insights and industry trends and challenges. The expert editions on various heat treatment processes reflect the latest developments. -

Differences and Applications of Carburizing and Cabonitriding
DIFFERENCES AND APPLICATIONS OF CARBURIZING AND CABONITRIDING Francisco GUANÍ, Especialidades Térmicas, S.A. de C.V. Fundidores 18 Zona Industrial Xhala Cuautitlán Izcalli, México, C.P. 54714 Carburizing Carburizado También conocido como Endurecimiento de la Capa. Also referred as Case Hardening. Es un tratamiento térmico que produce Is a heat treatment process that una superficie la cual es resistente al produces a surface which is resistant to desgaste, manteniendo la tenacidad y wear, while maintaining toughness and resistencia en el núcleo. strength of the core. Este tratamiento se aplica a partes de This treatment is applied to low carbon acero bajo carbono después de su steel parts after machining, as well as maquinado, también como a aceros de high alloy steel bearings, gears, and rodamiento de alta aleación, engranes y other components. otros componentes. Carburizing increases strength and El carburizado incrementa la tensión y la wear resistance by diffusing carbon resistencia al desgaste, mediante la into the surface of the steel creating a difusión de carbono en la superficie del case while retaining a substantially acero, creando una capa, mientras que el lesser hardness in the core. This núcleo mantiene una menor dureza. Este treatment is applied to low carbon tratamiento se aplica a aceros bajo steels after machining. carbono después de su maquinado. Strong and very hard-surface parts of Partes con superficies duras de formas intricate and complex shapes can be intricadas y complejas se pueden made of relatively lower cost materials obtener a costos relativamente bajos que that are readily machined or formed ya han sido maquinados o formados prior to heat treatment. -

Carbonitriding—Fundamentals, Modeling and Process Optimization
Carbonitriding—Fundamentals, Modeling and Process Optimization Report 13-02 Research Team: Liang He [email protected] Yuan Xu [email protected] Xiaolan Wang [email protected] Mei Yang [email protected] (508) 353-8889 Richard D. Sisson, Jr. [email protected] (508) 831-5335 Focus group: 1. Project Statement Objectives Develop a fundamental understanding of the process in terms of: • The diffusion of nitrogen in the steel depends on the nitrogen gradients as well as the carbon and other alloying element concentrations and gradients. The carbon content and gradients will have a significant influence on the nitrogen activity and therefore diffusivities. A fundamental understanding of the thermodynamics of solutions of carbon and nitrogen in alloyed austenite is necessary as well as the effects on diffusion of carbon and nitrogen. • The effect of nitrogen on the hardenability of carburized steels will be determined. Nitrogen stabilizes austenite and increases the hardenability by moving the TTT diagram to longer times. The effects of nitrogen content on these phenomena will be determined. • Determine the process control requirements for Carbonitriding in terms of temperature, atmosphere composition measurement and times based on the precision and accuracy required to control the process. Develop a computational model to determine the carbon and nitrogen concentration profiles in the steel in terms of temperature, atmosphere composition (ENDOGAS + NH3), steel surface 1 condition, and alloy composition. This tool will be designed to predict the carbon and nitrogen profiles based on the input of the process parameters of temperature (time), and atmosphere composition (time). Boost and diffuse cycles will be included. • CarbTool© will be modified to create CarboNitrideTool©, a software that will simulate the carbonitriding process, by adding the nitrogen absorption and diffusion in austenite with concomitant carbon uptake and diffusion. -

CDA 681 Bronze Brazing Alloy
Technical Data Sheet CDA 681 Bronze Brazing Alloy NOMINAL COMPOSITION Copper 58.0% ± 2.0% Zinc Remainder Iron 0.75% ± 0.45% Tin 0.95% ± 0.15% Manganese 0.01% - 0.50% Lead 0.05% Aluminum 0.01% Silicon 0.04% - 0.15% Other Elements (Total) 0.50% Max PHYSICAL PROPERTIES Color Brass Yellow Melting Point (Solidus) 1590°F (866°C) Flow Point (Liquidus) 1630°F (888°C) Brazing Temperature Range 1670°F - 1750°F (910°C - 954°C) Specific Gravity 8.07 Density (Lbs /in3) 0.292 Electrical Conductivity (%IACS) (1) 24.0 Electrical Resistivity (Microhm-cm) 7.18 (1) IACS = International Annealed Copper Standard PRODUCT USES CDA 681 is a low fuming bronze filler metal used for brazing of ferrous and non-ferrous alloys such steel and copper. This alloy is typically used where close fit up cannot be maintained and high brazing temperatures are permissible. BRAZING CHARACTERISTICS CDA 681 has a good wetting characteristics on ferrous and non-ferrous materials particularly steels and coppers. Maximum strength and joint integrity are obtained where joint clearance falls within the range of 0.003 in. - 0.005 in. per side. Handy Flux Hi-Temp should be used in conjunction with this alloy. Heating methods include torch, induction and furnace. PROPERTIES OF BRAZED JOINTS The properties of a brazed joint are dependent upon numerous factors including base metal properties, joint design, metallurgical interaction between the base metal and the filler metal. AVAILABLE FORMS Wire, engineered preforms, specialty preforms per customer specification. January 2017 CDA 681 Page 1 of 2 Lucas-Milhaupt, Inc. -

Enghandbook.Pdf
785.392.3017 FAX 785.392.2845 Box 232, Exit 49 G.L. Huyett Expy Minneapolis, KS 67467 ENGINEERING HANDBOOK TECHNICAL INFORMATION STEELMAKING Basic descriptions of making carbon, alloy, stainless, and tool steel p. 4. METALS & ALLOYS Carbon grades, types, and numbering systems; glossary p. 13. Identification factors and composition standards p. 27. CHEMICAL CONTENT This document and the information contained herein is not Quenching, hardening, and other thermal modifications p. 30. HEAT TREATMENT a design standard, design guide or otherwise, but is here TESTING THE HARDNESS OF METALS Types and comparisons; glossary p. 34. solely for the convenience of our customers. For more Comparisons of ductility, stresses; glossary p.41. design assistance MECHANICAL PROPERTIES OF METAL contact our plant or consult the Machinery G.L. Huyett’s distinct capabilities; glossary p. 53. Handbook, published MANUFACTURING PROCESSES by Industrial Press Inc., New York. COATING, PLATING & THE COLORING OF METALS Finishes p. 81. CONVERSION CHARTS Imperial and metric p. 84. 1 TABLE OF CONTENTS Introduction 3 Steelmaking 4 Metals and Alloys 13 Designations for Chemical Content 27 Designations for Heat Treatment 30 Testing the Hardness of Metals 34 Mechanical Properties of Metal 41 Manufacturing Processes 53 Manufacturing Glossary 57 Conversion Coating, Plating, and the Coloring of Metals 81 Conversion Charts 84 Links and Related Sites 89 Index 90 Box 232 • Exit 49 G.L. Huyett Expressway • Minneapolis, Kansas 67467 785-392-3017 • Fax 785-392-2845 • [email protected] • www.huyett.com INTRODUCTION & ACKNOWLEDGMENTS This document was created based on research and experience of Huyett staff. Invaluable technical information, including statistical data contained in the tables, is from the 26th Edition Machinery Handbook, copyrighted and published in 2000 by Industrial Press, Inc.