Visual Constraints Upon Avian Behaviour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Light and Electron Microscopy Study of the Pecten Oculi of the Jungle Crow (Corvus Macrorhynchos)
Okajimas Folia Anat.Pecten Jpn., 87oculi(3): of 75–83, the jungle November, crow 201075 Light and electron microscopy study of the pecten oculi of the Jungle Crow (Corvus macrorhynchos) By Mohammad Lutfur RAHMAN1, 2, Eunok LEE1, Masato AOYAMA1 and Shoei SUGITA1 1 Department of Animal Science, Faculty of Agriculture, Utsunomiya University, Utsunomiya, Japan 2 Department of Anatomy and Histology, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulsi, Chittagong, Bangladesh –Received for Publication, February 3, 2010– Key Words: jungle crow, pecten oculi Summary: In this study, the pecten oculi of a diurnally active bird, the Japanese jungle crow (Corvus macrorhynchos), was examined using light and electron microscopy. In this species, the pecten consisted of 24–25 highly vascularized pleats held together apically by a heavily pigmented ‘bridge’ and projected freely into the vitreous body in the ventral part of the eye cup. Ascending and descending blood vessels of varying caliber, together with a profuse network of capillaries, essentially constituted the vascular framework of the pecten. A distinct distribution of melanosomes was discernible on the pecten, the concentration being highest at its apical end, moderate at the crest of the pleats and lowest at the basal and lateral margins. Overlying and within the vascular network, a close association between blood vessels and melanocytes was evident. It is conjectured that such an association may have evolved to augment the structural reinforcement of this nutritive organ in order to keep it firmly erectile within the gel-like vitreous. Such erectility may be an essential prerequisite for its optimal functioning as well as in its overt use as a protective shield against the effects of ultraviolet light, which otherwise might lead to damage of the pectineal vessels. -

Gross, Histomorphological, Histochemical and Ultrastruc- Tural
Mishra P and Meshram, Archiv Zool Stud 2019, 2: 009 DOI: 10.24966/AZS-7779/100009 HSOA Archives of Zoological Studies Original Article Keywords: Gross study; Guinea fowl; Histochemistry; Histomor- Gross, Histomorphological, phology; Pecten oculi; Ultrastructure Histochemical and Ultrastruc- Introduction tural Studies of Pecten Oculi in Hemelted guinea fowl (Numida meleagris), the best known of the Guinea Fowl (Numida Meleagris) guinea fowl family Numididae having its origin in Africa and has been widely introduced into West Indies, Brazil, Australia and India. Pratiksha Mishra and Balwant Meshram* It is raised as a pet or the bird for meat which is without superfluous fat than chicken and has marginally more protein than turkey meat, Department of Veterinary Anatomy and Histology, College of Veterinary roughly half the fat of chicken [1]. There are several components and Animal science, Rajasthan, India which bought into being the bird eye, but pecten, a unique comb like structure is one of them which is specifically found in avian eye and Abstract some reptiles [2]. Pecten Oculi of Guinea fowl (Numida meleagris) was studied on The pecten oculi is highly vascular and pigmented structure which their 18 eyes for gross, histological, histochemical and ultrastructural plays an important role in various functions of the eye such as provid- observations. The pecten oculi has 13 to 17 number of accordion ing nutrition to retina, maintaining retinal circulation, regulation of (pectineal) folds. These accordion folds were initiated from cauda intraocular pressure, maintaining the pH and providing oxygen gra- of optic nerve and travelled via fundus distally into the vitreous hu- dient to retina [3]. -
Dwarf Thistle, Cirsi
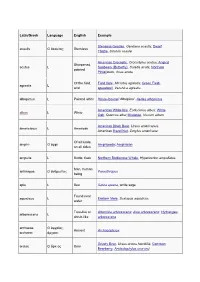
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

The Effect of Intense Light on Bird Behavior and Physiology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Wildlife Damage Management, Internet Center Bird Control Seminars Proceedings for October 1973 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Ohio State University Follow this and additional works at: https://digitalcommons.unl.edu/icwdmbirdcontrol Part of the Environmental Sciences Commons Lustick, Sheldon, "THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY" (1973). Bird Control Seminars Proceedings. 119. https://digitalcommons.unl.edu/icwdmbirdcontrol/119 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Bird Control Seminars Proceedings by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 171 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Zoology Department Ohio State University , Columbus , Ohio 43210 It has been known for centuries that light (photoperiod ) is possibly the major environmental stimuli affecting bird behavior and physiology. The length of the light period stimulates the breeding cycle , migration , fat de - position , and molt in most species of birds. Therefore , it is only natural that one would think of using light as a means of bird control. In fa ct , light has already been used as a bird control; flood -light traps have been used to trap blackbirds (Meanley 1971 ); Meanley states that 2000 -W search lights have been used to alleviate depredation by ducks in rice fields. -

Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174656. doi:10.1242/jeb.174656 RESEARCH ARTICLE Magnificent magpie colours by feathers with layers of hollow melanosomes Doekele G. Stavenga1,*, Hein L. Leertouwer1 and Bodo D. Wilts2 ABSTRACT absorption coefficient throughout the visible wavelength range, The blue secondary and purple-to-green tail feathers of magpies are resulting in a higher refractive index (RI) than that of the structurally coloured owing to stacks of hollow, air-containing surrounding keratin. By arranging melanosomes in the feather melanosomes embedded in the keratin matrix of the barbules. barbules in more or less regular patterns with nanosized dimensions, We investigated the spectral and spatial reflection characteristics of vivid iridescent colours are created due to constructive interference the feathers by applying (micro)spectrophotometry and imaging in a restricted wavelength range (Durrer, 1977; Prum, 2006). scatterometry. To interpret the spectral data, we performed optical The melanosomes come in many different shapes and forms, and modelling, applying the finite-difference time domain (FDTD) method their spatial arrangement is similarly diverse (Prum, 2006). This has as well as an effective media approach, treating the melanosome been shown in impressive detail by Durrer (1977), who performed stacks as multi-layers with effective refractive indices dependent on extensive transmission electron microscopy of the feather barbules the component media. The differently coloured magpie feathers are of numerous bird species. He interpreted the observed structural realised by adjusting the melanosome size, with the diameter of the colours to be created by regularly ordered melanosome stacks acting melanosomes as well as their hollowness being the most sensitive as optical multi-layers. -

Visual Adaptations of Diurnal and Nocturnal Raptors
Seminars in Cell and Developmental Biology 106 (2020) 116–126 Contents lists available at ScienceDirect Seminars in Cell & Developmental Biology journal homepage: www.elsevier.com/locate/semcdb Review Visual adaptations of diurnal and nocturnal raptors T Simon Potiera, Mindaugas Mitkusb, Almut Kelbera,* a Lund Vision Group, Department of Biology, Lund University, Sölvegatan 34, S-22362 Lund, Sweden b Institute of Biosciences, Life Sciences Center, Vilnius University, Saulėtekio Av 7, LT-10257 Vilnius, Lithuania HIGHLIGHTS • Raptors have large eyes allowing for high absolute sensitivity in nocturnal and high acuity in diurnal species. • Diurnal hunters have a deep central and a shallow temporal fovea, scavengers only a central and owls only a temporal fovea. • The spatial resolution of some large raptor species is the highest known among animals, but differs highly among species. • Visual fields of raptors reflect foraging strategies and depend on the divergence of optical axes and on headstructures • More comparative studies on raptor retinae (preferably with non-invasive methods) and on visual pathways are desirable. ARTICLE INFO ABSTRACT Keywords: Raptors have always fascinated mankind, owls for their highly sensitive vision, and eagles for their high visual Pecten acuity. We summarize what is presently known about the eyes as well as the visual abilities of these birds, and Fovea point out knowledge gaps. We discuss visual fields, eye movements, accommodation, ocular media transmit- Resolution tance, spectral sensitivity, retinal anatomy and what is known about visual pathways. The specific adaptations of Sensitivity owls to dim-light vision include large corneal diameters compared to axial (and focal) length, a rod-dominated Visual field retina and low spatial and temporal resolution of vision. -

Bird Dispersal Techniques Are a Vital Part of Safely and Droppings And, in Some Cases, Their Aggressive Behavior Efficiently Reducing Bird Conflicts with Humans
U.S. Department of Agriculture Animal & Plant Health Inspection Service Wildlife Services August 2016 Bird Dispersal Wildlife Damage Management Technical Series Techniques Thomas W. Seamans Supervisory Wildlife Biologist USDA-APHIS-Wildlife Services National Wildlife Research Center Sandusky, Ohio Allen Gosser State Director USDA-APHIS-Wildlife Services Rensselaer, New York Figure 1. Photo of a frightened wild turkey (Meleagris gallopavo). Human-Wildlife Conflicts Quick Links Conflicts between humans and birds likely Successful dispersal techniques should have existed since agricultural practices capitalize on bird sensory capabilities. If Human-Wildlife Conflicts 1 began. Paintings from ancient Greek, birds cannot perceive the dispersal Habitat Modification 2 Egyptian, and Roman civilizations depict technique, it will not be effective in Frightening Techniques 4 birds attacking crops. In Great Britain, dispersing birds. recording of efforts at reducing bird Bird Management 7 damage began in the 1400s, with books Birds rely primarily on their vision and Examples on bird control written in the 1600s. Even hearing to find food, avoid predators, and Conclusion 8 so, the problem persists. Avian damage to locate mates. Bird vision is quite different crops remains an issue today, but we also from human vision; birds can see colors Glossary & Key Words 10 are concerned with damage to homes, that humans cannot perceive (including Resources 11 businesses, and aircraft, and the the ultraviolet range), and and they detect possibility of disease transmission from and use polarized light. Bird response to birds to humans or livestock. scare devices (Figure 1) that rely on vision Page 2 WDM Technical Series—Bird Dispersal may depend on the visibility of the object to the bird, as present. -

Imaging of Physiological Retinal Structures in Various Raptor Species Using Optical Coherence Tomography (Oct)
Aus dem Zentrum für klinische Tiermedizin Tierärztliche Fakultät der Ludwig – Maximilians - Universität München Arbeit angefertigt unter Leitung von Prof. Dr. R. Korbel IMAGING OF PHYSIOLOGICAL RETINAL STRUCTURES IN VARIOUS RAPTOR SPECIES USING OPTICAL COHERENCE TOMOGRAPHY (OCT) Inaugural - Dissertation zur Erlangung der tiermedizinischen Doktorwürde der Tierärztlichen Fakultät der Ludwig – Maximilians - Universität München vorgelegt von María Luisa Velasco Gallego aus Valladolid München 2015 Aus dem Zentrum für klinische Tiermedizin der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München Lehrstuhl für aviäre Medizin und Chirurgie Arbeit angefertigt unter der Leitung von Prof. Dr. R. Korbel Mitbetreuung durch: Priv.-Doz. Dr. Monika Rinder Gedruckt mit Genehmigung der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München Dekan: Univ.-Prof. Dr. Joachim Braun Berichterstatter: Univ.-Prof. Dr. Rüdiger T. Korbel Korreferent/en: Priv.-Doz. Dr. Sven Reese Tag der Promotion: 31. Januar 2015 A mi querida familia y a Edu INDEX INDEX .......................................................................................................................... V LIST OF ABBREVIATIONS .......................................................................................... IX 1 INTRODUCTION ................................................................................................ 1 2 LITERATURE ..................................................................................................... 3 2.1 Optical Coherence -

Plumage and Behavioral Development of Nestling White Ibises
Wilson Bull., 102(2), 1990, pp. 226-238 PLUMAGE AND BEHAVIORAL DEVELOPMENT OF NESTLING WHITE IBISES TONI L. DE SANTO,’ SUSAN G. MCDOWELL,~ AND KEITH L. BILDSTEIN~ Ans-rticr.- We describe the physical characteristicsand behavioral development OI 17 hand-reared and more than 400 parent-reared nestling White Ibises (Eudocimus albus) hatched in 1985 through 1988 at Pumpkinseed Island, a large colony site in coastal South Carolina. Hatchling ibises are covered with a Pale Neutral Gray to Jet Black natal plumage. About 30% of the hatchlingspossess a tuft of white feathers on their crown, and this pattern persiststhroughout the nestling period. Juvenal plumage, which is complete by 60 days, is mainly Vandyke Brown and Blackish Neutral Gray dorsally and creamy white ventrally. The bill, which is straight at hatching, begins to curve downward at about 14 days. Nestling White Ibises exhibit considerableindividual variation in bill markings from approximately 10 days of age through fledging. Increasingly persistent beggingvocalizations begin within hours of hatching. Nestlings walk on partially extended legsat eight days of age, pirate food from other nestlingsand form crechesat 2 1 days of age, and fledge and join all juvenile and mixed-age feeding flocks at 45-55 days of age. We suggestthat the phenotypic variability in plumage, bill coloration, and beggingcalls we describeenables parental ibises to identify more easily their offspringat the colony site. Received27 Feb. 1989, accepted12 Nov. 1989. Although the plumage and behavioral development of several species of wading birds has been studied in considerable detail (e.g., Hammerkops [&opus umbretta], Wilson et al. 1988; storks, Kahl 1962, 1966; Thomas 1984; herons, Gross 1923; Gavin0 and Dickerman 1972; Juarez and Dickerman 1972; Mc Vaugh 1972,1975; Snow 1974; Merritt 198 l), there are few detailed studies of juvenile ibises. -

The Dulmison BFD Is Available in a Variety of Colors and Different Sizes to Accommodate Wires Ranging from 0.175 to 1.212 Inches (Figure 4-9)
The Dulmison BFD is available in a variety of colors and different sizes to accommodate wires ranging from 0.175 to 1.212 inches (Figure 4-9). The BFD has been effective when tested on transmission overhead static wires in Europe, where typical spacing ranges &om 16 to 33 feet. In North America, the BFD also has shown to be effective in reducing waterfowl collisions with overhead static wires (Crowder 2000). The BFD is believed to be effective because its profile increases line visibility. As with “active devices” such as the Flapper, these more “passive” devices have not been tested on communication tower guy wires; however, it is assumed that they would Figure 4-9 Bird Flight Diverters for Small and Larger increase the profile and, therefore, the Wires visibility of the guy wires during daytime conditions. Regarding long-term use, BFD colors may fade after long periods of exposure but should not become brittle or lose their elastic properties. ESKOM has used the Preformed Line Products, BFD in South Atka for years with no reports of mechanical failure (van Rooyen 2000) although some red PVC devices have faded. 4.2.1.4 Swan Flight Diverter The Swan Flight Diverter (SFD) is similar to the BFD but includes four 7-inch spirals (Figure 4-10). The SFD also is made from a high-impact, standard gray PVC and is UV stabilized. The Dulmison SFD is available in a variety of colors and sizes to accommodate wires ranging fiom 0.175 to 1.212 inches. Notice Of Inquiry Comment Review 4-14 September 2004 Final Figure 4-10 Swan Flight Diverters Being Placed on a Static Wire As with the BFD, the SFD has been shown to be effective when installed on transmission overhead static wires in North America, but has not been tested on tower guy wires. -

The Unsuspected Intrinsic Property of Melanin to Dissociate the Water Molecule
MOJ Cell Science & Report Research Article Open Access Towards a new ophthalmic biology and physiology: the unsuspected intrinsic property of melanin to dissociate the water molecule Abstract Volume 4 Issue 2 - 2017 The development and evolution of eyes is ancient and difficult problem in biology. Arturo Solis Herrera Darwin postulated a prototype eye which can evolve under natural selection. Neo- Director, Human Photosynthesis® Research Center, México Darwinists, based in morphological criteria, have postulated a polyphyletic origin that has evolved independently in the various animal phyla. Molecular phylogenetic Correspondence: Arturo Solis Herrera, Director, Human analyses and developmental genetic experiments, cast serious doubts on both Photosynthesis® Research Center, México, theories. The study of the development of the eye in the amphibian embryo has been Email [email protected] a formidable tool research to experimental embryology and evolutionary biology as early as 1901. The embryonic induction, the interactions between different embryonic Received: March 07, 2017 | Published: April 27, 2017 tissues (organizers); and the role of genes, were conceived as result of reciprocal transplantation experiments. The classical description is that the eye in vertebrates develops from the neural plate, as an evagination from the brain, forming the optic vesicle; which subsequently invaginates to form the optic cup. The inner layer of the optic cup forms the retina with its photoreceptor layers, whereas the outer layer gives rise to the pigment epithelium which absorbs the light in the back of the retina. However, failed to recognize the importance of melanin pigment. The mammalian eye consists of several layers that contain melanin, 40 % more than skin in average. -

November 2019 (Except December), at the Hal Holmes Center Next to the Ellensburg Public Library, Are Open to the Public
All Audubon meetings, held on the 3rd Thursday of each month, 7:00pm, September through May November 2019 (except December), at the Hal Holmes Center next to the Ellensburg Public Library, are open to the public. Please come and meet with us, and stay afterwards for juice, treats, and conversation. Many thanks to the Ellensburg Public Library for sponsoring our meetings! 7:00pm • November 21st • General Program There’s a Fungus Among Us! What lurks beneath us on earth’s carpet? The mission of Can I safely eat that? What purpose does Kittitas Audubon that organism serve? is to develop an appreciation of Find out at November’s program on the 21st! nature through Helen Lau will introduce the audience to the secret life of fungi and discuss some of their ecological functions. She will share images of some of our most common education and local fungi and common edible fungi as well as their look-a-likes. Helen Lau is a conservation, botanist for the USFS on the Okanogan-Wenatchee National Forest. She manages with a focus on the rare botanical species (plants, lichens, bryophytes and fungi), native plant restoration and invasive plant program on the Cle Elum Ranger District. Helen’s birds. research interests are in fungi biodiversity and she received an undergraduate degree at Evergreen State College and her master’s degree on mycorrhizae ecology in the Biological Sciences Department at Central Washington University. Photos provided by Helen Lau The Hooter • Kittitas Audubon’s Newsletter kittitas audubon • www.kittitasaudubon.org By Gloria Baldi Following is a brief summary of the September 5th Board minutes: Board Members Announcements • Injured birds from Kittitas County have previously been taken to Blue Mountain Wildlife in Pendleton, PRESIDENT Oregon.