National Chemicals Management Profile MONGOLIA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

University of Groningen Reduction of Aldehydes and Ketones by Sodium Dithionite Vries, Johannes G. De
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Groningen University of Groningen Reduction of Aldehydes and Ketones by Sodium Dithionite Vries, Johannes G. de; Kellogg, Richard M. Published in: Journal of Organic Chemistry DOI: 10.1021/jo01309a011 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 1980 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Vries, J. G. D., & Kellogg, R. M. (1980). Reduction of Aldehydes and Ketones by Sodium Dithionite. Journal of Organic Chemistry, 45(21), 4126-4129. https://doi.org/10.1021/jo01309a011 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 12-11-2019 4126 J. Org. Chem. 1980,45,4126-4129 Reduction of Aldehydes and Ketones by Sodium Dithionite Johannes G. -

The Journal of Organic Chemistry 1959 Volume 24 No.8
THE JOURNAL OF Organic Chemistry © Copyright 1959 Volume 24, Number 8 by the American Chemical Society August 31, 1959 [Contribution from th e C hem istry D epartm ent of Syracuse U n iversity ] Condensation of Thiophenols and Formaldehyde with Some Aromatic Amines GERALD F. GRILLOT and ROBERT E. SCHAFFRATH Received September 85, 1958 N-Arylaminomethyl aryl sulfides and l,3,5-triaryl-l,5-dithia-3-azapentanes have been prepared by condensing primary aromatic amines with formaldehyde and thiophenols. A-Methylanilines condense with formaldehyde and thiophenols to form Ar-methyl-Ar-arylaminomethyl aryl sulfides. Two arylaminoethy 1 aryl sulfides were prepared by condensing 0-chloroethylaniline with the sodium salt of the thiophenol. Basicities of these arylaminoalkyl aryl sulfides have been related to (a) the presence of electrophilic substituents attached to the aryl groups and (b) the number of carbon atoms separating the nitrogen and sulfur atoms. Recently Grillot et a l.1 have demonstrated that Whereas primary aliphatic amines generally the thiophenols condense with secondary aliphatic give a mixture of secondary and tertiary amines in amines and formaldehyde to form dialkylamino- the Mannich reaction, primary aromatic amines methyl aryl sulfides similar in structure to the can be condensed with thiophenols and formalde dialkylaminomethyl alkyl sulfides prepared by hyde in a 1:1:1 mole ratio to give almost exclusively McLeod and Robinson2 3by condensing aliphatic moderately stable usually crystalline W-arylamino- mercaptans with aliphatic -

Ereztech LLC P3553 Safety Data Sheet
EREZTECH LLC 11555 Medlock Bridge Road, Suite 100, Johns Creek, GA 30097, USA T: +1.888.658.1221 F: 1.678.619.2020 E: [email protected] W: https://ereztech.com Section 1. Identification Product Name: Phosphorus trifluoride Product Type: Gas CAS Number: 7783-55-3 Product Number: P3553 Product Manufacturer: Ereztech LLC 11555 Medlock Bridge Road, Suite 100 Johns Creek, GA 30097 Product Information: (888) 658-1221 In Case of an Emergency: CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International); CCN836180 *** Contact manufacturer for all non-emergency calls. Section 2. Hazards Identification Appearance/Odor: Colorless, odorless gas. Classification: ACUTE TOXICITY, ORAL - Category 4, H302 ACUTE TOXICITY, DERMAL - Category 4, H312 SKIN CORROSION/IRRITATION - Category 1B, H314 SERIOUS EYE DAMAGE/EYE IRRITATION - Category 2, H318 ACUTE TOXICITY, INHALATION - Category 1, H330 SPECIFIC TARGET ORGAN TOXICITY, SINGLE EXPOSURE; RESPIRATORY TRACT IRRITATION – Category 3, H335 GHS Label Elements Hazard Pictograms: Signal Word: DANGER Hazard Statements: H302: Harmful if swallowed. H312: Harmful in contact with skin. H314: Causes severe skin burns and eye damage. H318: Causes serious eye damage. H330: Fatal if inhaled. H335: May cause respiratory irritation. Ereztech P3553 Page 1 of 13 Revision: 1.00 Date of Issue: 11/27/2020 Phosphorus trifluoride Safety Data Sheet Section 2. Hazards Identification Precautionary Statements Prevention: P260: Do not breathe fumes/gases/mists/vapors/sprays. P264: Wash skin thoroughly after handling. P270: Do not eat, drink or smoke when using this product. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/ protective clothing/ eye protection/ face protection. -
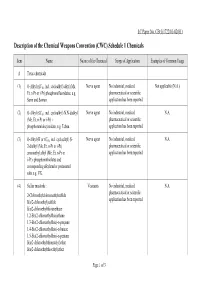
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Calculation of Properties of Environmental Interest
1 Journal of Environmental Monitoring 10:435-442, 2008 Solvation parameters for mercury and mercury(II) compounds: calculation of properties of environmental interest Michael H. Abraham, *a Javier Gil-Lostes, a William E. Acree, Jr. b J. Enrique Cometto-Muñiz, c and William S. Cain. c a Department of Chemistry, University College London, 20 Gordon Street, London WC1H OAJ, UK. Email: [email protected] b Department of Chemistry, P. O. Box 305070, University of North Texas, Denton, TX 76203-5070, USA. c Chemosensory Perception Laboratory, Department of Surgery (Otolaryngology), University of California, San Diego, La Jolla, CA 92093-0957, USA 2 Descriptors have been determined for four inorganic mercury(II) species and for seventeen organic mercury(II) species, using experimental literature data. These descriptors can then be used in equations that we have already set out in order to estimate a large number of physicochemical properties. These include the water to octanol partition coefficient and the gas to water partition coefficient. For the organic mercury(II) species, including dimethylmercury and the methylmercury(II) halides, the latter has been estimated over the temperature range 273-373K. Introduction Mercury and mercury(II) compounds are important species; dimethylmercury and methylmercury(II) compounds are known environmental pollutants. Although a number of thermodynamic properties are available especially for mercury 1, 2 and mercury(II) halides, 2 many other properties that are relevant to environmental and health issues are not known. A number of computational methods can be used for the calculation of a range of properties of compounds, but many of these methods such as SPARC,3 Advanced Chemistry Development 4 and PharmaAlgorithms, 5 cannot deal with any compounds that contain mercury atoms. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

(12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub
US 20050044778A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub. Date: Mar. 3, 2005 (54) FUEL COMPOSITIONS EMPLOYING Publication Classification CATALYST COMBUSTION STRUCTURE (51) Int. CI.' ........ C10L 1/28; C1OL 1/24; C1OL 1/18; (76) Inventor: William C. Orr, Denver, CO (US) C1OL 1/12; C1OL 1/26 Correspondence Address: (52) U.S. Cl. ................. 44/320; 44/435; 44/378; 44/388; HOGAN & HARTSON LLP 44/385; 44/444; 44/443 ONE TABOR CENTER, SUITE 1500 1200 SEVENTEENTH ST DENVER, CO 80202 (US) (57) ABSTRACT (21) Appl. No.: 10/722,127 Metallic vapor phase fuel compositions relating to a broad (22) Filed: Nov. 24, 2003 Spectrum of pollution reducing, improved combustion per Related U.S. Application Data formance, and enhanced Stability fuel compositions for use in jet, aviation, turbine, diesel, gasoline, and other combus (63) Continuation-in-part of application No. 08/986,891, tion applications include co-combustion agents preferably filed on Dec. 8, 1997, now Pat. No. 6,652,608. including trimethoxymethylsilane. Patent Application Publication Mar. 3, 2005 US 2005/0044778A1 FIGURE 1 CALCULATING BUNSEN BURNER LAMINAR FLAME VELOCITY (LFV) OR BURNING VELOCITY (BV) CONVENTIONAL FLAME LUMINOUS FLAME Method For Calculating Bunsen Burner Laminar Flame Velocity (LHV) or Burning Velocity Requires Inside Laminar Cone Angle (0) and The Gas Velocity (Vg). LFV = A, SIN 2 x VG US 2005/0044778A1 Mar. 3, 2005 FUEL COMPOSITIONS EMPLOYING CATALYST Chart of Elements (CAS version), and mixture, wherein said COMBUSTION STRUCTURE element or derivative compound, is combustible, and option 0001) The present invention is a CIP of my U.S. -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Effects of Inorganic Mercury on Developing Zebrafish (Danio Rerio) Larvae
EFFECTS OF INORGANIC MERCURY ON DEVELOPING ZEBRAFISH (DANIO RERIO) LARVAE A Thesis Submitted to the College of Graduate Studies and Research In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In the Toxicology Graduate Program University of Saskatchewan Saskatoon By TRACY CLARYLINDA MACDONALD Copyright Tracy Clarylinda MacDonald, September, 2015. All rights reserved. PERMISSION TO USE STATEMENT In presenting this thesis in partial fulfillment of the requirements for a postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Chair of the Toxicology Graduate Program Toxicology Centre University of Saskatchewan 44 Campus Drive Saskatoon, Saskatchewan, Canada S7N 5B3 i ABSTRACT Mercury (Hg) compounds are some of the most toxic compounds of any heavy metal on earth.