The Dissolution of Gold by Selenic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
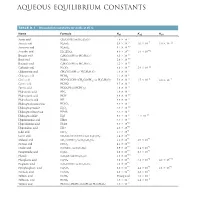
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -

Acids and Bases
Name Date Class CHAPTER 14 REVIEW Acids and Bases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Name the following compounds as acids: sulfuric acid a. H2SO4 sulfurous acid b. H2SO3 hydrosulfuric acid c. H2S perchloric acid d. HClO4 hydrocyanic acid e. hydrogen cyanide 2. H2S Which (if any) of the acids mentioned in item 1 are binary acids? 3. Write formulas for the following acids: HNO2 a. nitrous acid HBr b. hydrobromic acid H3PO4 c. phosphoric acid CH3COOH d. acetic acid HClO e. hypochlorous acid 4. Calcium selenate has the formula CaSeO4. H2SeO4 a. What is the formula for selenic acid? H2SeO3 b. What is the formula for selenous acid? 5. Use an activity series to identify two metals that will not generate hydrogen gas when treated with an acid. Choose from Cu, Ag, Au, Pt, Pd, or Hg. 6. Write balanced chemical equations for the following reactions of acids and bases: a. aluminum metal with dilute nitric acid ϩ → ϩ 2Al(s) 6HNO3(aq) 2Al(NO3)3(aq) 3H2(g) b. calcium hydroxide solution with acetic acid ϩ → ϩ Ca(OH)2(aq) 2CH3COOH(aq) Ca(CH3COO)2(aq) 2H2O(l ) MODERN CHEMISTRY ACIDS AND BASES 117 Copyright © by Holt, Rinehart and Winston. All rights reserved. Name Date Class SECTION 1 continued 7. Write net ionic equations that represent the following reactions: a. the ionization of HClO3 in water ϩ → ϩ ϩ Ϫ HClO3(aq) H2O(l ) H3O (aq) ClO3 (aq) b. NH3 functioning as an Arrhenius base ϩ → ϩ ϩ Ϫ NH3(aq) H2O(l ) ← NH4 (aq) OH (aq) 8. -

Underactive Thyroid
Underactive Thyroid PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information. PDF generated at: Thu, 21 Jun 2012 14:27:58 UTC Contents Articles Thyroid 1 Hypothyroidism 14 Nutrition 22 B vitamins 47 Vitamin E 53 Iodine 60 Selenium 75 Omega-6 fatty acid 90 Borage 94 Tyrosine 97 Phytotherapy 103 Fucus vesiculosus 107 Commiphora wightii 110 Nori 112 Desiccated thyroid extract 116 References Article Sources and Contributors 121 Image Sources, Licenses and Contributors 124 Article Licenses License 126 Thyroid 1 Thyroid thyroid Thyroid and parathyroid. Latin glandula thyroidea [1] Gray's subject #272 1269 System Endocrine system Precursor Thyroid diverticulum (an extension of endoderm into 2nd Branchial arch) [2] MeSH Thyroid+Gland [3] Dorlands/Elsevier Thyroid gland The thyroid gland or simply, the thyroid /ˈθaɪrɔɪd/, in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage (which forms the laryngeal prominence, or "Adam's apple"). The isthmus (the bridge between the two lobes of the thyroid) is located inferior to the cricoid cartilage. The thyroid gland controls how quickly the body uses energy, makes proteins, and controls how sensitive the body is to other hormones. It participates in these processes by producing thyroid hormones, the principal ones being triiodothyronine (T ) and thyroxine which can sometimes be referred to as tetraiodothyronine (T ). These hormones 3 4 regulate the rate of metabolism and affect the growth and rate of function of many other systems in the body. T and 3 T are synthesized from both iodine and tyrosine. -

Process for the Preparation of Chacogens and Chalcogenide Alloys of Controlled Average Crystallite Size
Europaisches Patentamt European Patent Office @ Publication number: 0 1 60 493 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 85302825.6 © Int. CI.4: C 01 B 19/02 _ C 01 B 19/04 © Date of filing: 23.04.85 © Priority: 23.04.84 US 603019 © Applicant: XEROX CORPORATION Xerox Square - 020 Rochester New York 14644(US) @ Date of publication of application: 06.11.85 Bulletin 85/45 @ Inventor: Badesha, Santokh Singh 665 Cievenger Road @ Designated Contracting States: Ontario New York 1451 9(US) DE FR GB © Inventor: Fekete, George Thomas 111 Dale Road Rochester New York 14625IUS) © Representative: Goode, Ian Roy et al, European Patent Attorney do Rank Xerox Limited Patent Department Rank Xerox House 338 Euston Road London NW1 3BH(GB) (64) Process for the preparation of chacogens and chalcogenide alloys of controlled average crystallite size. A process for the preparation of chalcogens and chal- cogenide alloys which comprises subjecting the correspond- ing esters to a reduction or coreduction reaction, for example using hydrazine, at a temperature between about 40°C and 135°C. The morphology/crystallite size of the product is controlled by suitably selecting a temperature within this range, depending on the particular chalcogen or alloy being prepared. The product is either a non-crystalline material, or has an average crystallite size between about 15 and 52 nm. This invention relates to a process for the preparation of chalcogens and chalcogenide alloys, the process being of the kind which comprises subjecting the corresponding esters to a reduction or coreduction reaction. Such a process for preparing high purity tellurium is described in EP-A-0 102 753, and such a process for preparing high purity chalcogenide alloys, such as selenium/tellurium alloys, is described in EP-A-0 101 238. -

1,2-Cyclohexanedione Dioxime
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

(12) United States Patent (10) Patent No.: US 8,524,796 B2 Kim Et Al
US008524796B2 (12) United States Patent (10) Patent No.: US 8,524,796 B2 Kim et al. (45) Date of Patent: *Sep. 3, 2013 (54) ACTIVE POLYMER COMPOSITIONS WO 20060967.91 9, 2006 WO 2007024.125 3, 2007 (75) Inventors: Young-Sam Kim, Midland, MI (US); WO 20070785682007030791 3,7/2007 2007 Leonardo C. Lopez, Midland, MI (US); WO 2007099397 9, 2007 Scott T. Matteucci, Midland, MI (US); WO 2007121458 10/2007 Steven R. Lakso, Sanford, MI (US) WO 2008101051 8, 2008 WO 2008112833 9, 2008 (73) Assignee: Dow Global Technologies LLC WO 2008.150970 12/2008 WO 2009 134824 11, 2009 (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 U.S.C. 154(b) by 457 days. Ciferri, Alberto, “Supramolecular Polymers'. Second Edition, 2005, pp. 157-158, CRC Press. This patent is Subject to a terminal dis Corbin et al., “Chapter 6 Hydrogen-Bonded Supramolecular Poly claimer. mers: Linear and Network Polymers and Self-Assembling Discotic Polymers'. Supramolecular Polymers, 2nd edition, CRC Press, (21) Appl. No.: 12/539,793 2005, pp. 153-185. Duan et al., “Preparation of Antimicrobial Poly (e-caprolactone) (22) Filed: Aug. 12, 2009 Electrospun Nanofibers Containing Silver-Loaded Zirconium Phos phate Nanoparticles”, Journal of Applied Polymer Sciences, 2007. (65) Prior Publication Data vol. 106, pp. 1208-1214, Wiley Periodicals, Inc. US 201O/OO41292 A1 Feb. 18, 2010 Hagewood, "Potential of Polymeric Nanofibers for Nonwovens and Medical Applications'. Fiberjournal.com, Feb. 26, 2008, 4 Pages, Related U.S. Application Data J.Hagewood, LLC and Ben Shuler, Hills, Inc. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Observations on the Rare Earths
];: H S ' . [bits sfi iiie &rc E*rlks emisiry M. S. I 9 1 5 J".J33i:tA23.V" THE UNIVERSITY OF ILLINOIS LIBRARY VMS 0F "' HE UNIVEHSITV OF UuuM OBSERVATIONS ON THE RARE EARTHS BY EDWARD WICHERS A.B. Hope College, 1913 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN CHEMISTRY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1915 Digitized by the Internet Archive in 2013 http://archive.org/details/observationsonraOOwich_0 UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL 5* i9X I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPER- VISION by gflwart Wlchera, entitled 0T> serrations on tue Bare Eartaa BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE nxr^r.^ ™? Master of Science in cnemistry DEGREE OF _ __ _ - _ _ _ In Charge of Thesis IflC // ac 7 ^ / Head of Department Recommendation concurred in :* Committee on Final Examination* *Required for doctor's degree but not for master's. 1 OBSERVATIONS ON IEB RARE EARTHS. INTRODUCTION. The chief problem which has stood in the way of the development of the chemistry of the rare earth metals has been that of finding adequate methods for the separation of the ele- ments comprising the group. For this reason, these elements have been, up to the present time, of little other than purely scien- tific interest. However, the comparatively recent discovery of many important industrial uses for other of the rarer elements, hitherto also considered of interest only in the laboratory, in- dicates the probability of finding for the rare earth metals uses which will be no less important. -

WATER CHEMISTRY CONTINUING EDUCATION PROFESSIONAL DEVELOPMENT COURSE 1St Edition
WATER CHEMISTRY CONTINUING EDUCATION PROFESSIONAL DEVELOPMENT COURSE 1st Edition 2 Water Chemistry 1st Edition 2015 © TLC Printing and Saving Instructions The best thing to do is to download this pdf document to your computer desktop and open it with Adobe Acrobat DC reader. Adobe Acrobat DC reader is a free computer software program and you can find it at Adobe Acrobat’s website. You can complete the course by viewing the course materials on your computer or you can print it out. Once you’ve paid for the course, we’ll give you permission to print this document. Printing Instructions: If you are going to print this document, this document is designed to be printed double-sided or duplexed but can be single-sided. This course booklet does not have the assignment. Please visit our website and download the assignment also. You can obtain a printed version from TLC for an additional $69.95 plus shipping charges. All downloads are electronically tracked and monitored for security purposes. 3 Water Chemistry 1st Edition 2015 © TLC We require the final exam to be proctored. Do not solely depend on TLC’s Approval list for it may be outdated. A second certificate of completion for a second State Agency $25 processing fee. Most of our students prefer to do the assignment in Word and e-mail or fax the assignment back to us. We also teach this course in a conventional hands-on class. Call us and schedule a class today. Responsibility This course contains EPA’s federal rule requirements. Please be aware that each state implements drinking water/wastewater/safety regulations may be more stringent than EPA’s or OSHA’s regulations. -

Selenium Recovery in Precious Metal Technology
Selenium Recovery in Precious Metal Technology Fabian Kling Willig Degree Project in Engineering Chemistry, 30 hp Report passed: February 2014 Supervisors: Tomas Hedlund, Umeå University Mikhail Maliarik, Outotec (Sweden) AB Abstract Selenium is mostly extracted from copper anode slimes because the selenium-rich ores are too rare to be mined with profit. When copper anode slimes are processed in a precious metal plant the first step is to remove copper by pressure leach in an autoclave. The anode slime is then dried and fed into a Kaldo furnace. The Kaldo is heated and reduction and smelting begins. During reduction, slag builders are added to form a slag with impurities and fluxes with coke breeze are added to reduce precious metal. In the oxidation step, selenium dioxide, sulphur dioxide and some tellurium dioxide are removed along with the process gas. The dioxides and the process gas are captured in a circulating venturi solution in the gas cleaning system. The dioxides lowers the pH of the venturi solution. Sodium hydroxide is added to the solution to keep pH above 4 in order to prevent selenium from precipitating in the circulation tank. After a Kaldo cycle, the venturi solution is transferred to a precipitation tank where sulphur dioxide is added in order to precipitate selenium. The precipitated selenium is finally collected in a filter press and sold as crude (99.5%) selenium. During commissioning of a precious metal plant and during processing of 2 batches anode slime, data such as pH and electrochemical potential was collected from the venturi solution and later shown in a Pourbaix diagram.