Understanding the Mechanism by Which Musa Balbisiana Resists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Basis of Disease Resistance in Banana Progenitor Musa Balbisiana Against Received: 25 September 2018 Accepted: 23 April 2019 Xanthomonas Campestris Pv
www.nature.com/scientificreports OPEN Molecular Basis of Disease Resistance in Banana Progenitor Musa balbisiana against Received: 25 September 2018 Accepted: 23 April 2019 Xanthomonas campestris pv. Published: xx xx xxxx musacearum Leena Tripathi 1, Jaindra Nath Tripathi1, Trushar Shah1, Kariuki Samwel Muiruri1 & Manpreet Katari2 Banana Xanthomonas wilt disease, caused by Xanthomonas campestris pv. musacearum (Xcm), is a major threat to banana production in east Africa. All cultivated varieties of banana are susceptible to Xcm and only the progenitor species Musa balbisiana was found to be resistant. The molecular basis of susceptibility and resistance of banana genotypes to Xcm is currently unknown. Transcriptome analysis of disease resistant genotype Musa balbisiana and highly susceptible banana cultivar Pisang Awak challenged with Xcm was performed to understand the disease response. The number of diferentially expressed genes (DEGs) was higher in Musa balbisiana in comparison to Pisang Awak. Genes associated with response to biotic stress were up-regulated in Musa balbisiana. The DEGs were further mapped to the biotic stress pathways. Our results suggested activation of both PAMP-triggered basal defense and disease resistance (R) protein-mediated defense in Musa balbisiana as early response to Xcm infection. This study reports the frst comparative transcriptome profle of the susceptible and resistant genotype of banana during early infection with Xcm and provide insights on the defense mechanism in Musa balbisiana, which can be used for genetic improvement of commonly cultivated banana varieties. Banana Xanthomonas wilt (BXW), caused by the bacterium Xanthomonas campestris pv. musacearum (Xcm), is one of the most devastating disease endangering the livelihood of millions of farmers in east Africa, which is the largest banana-producing and -consuming region in Africa1. -

SPECIAL PUBLICATION 6 the Effects of Marine Debris Caused by the Great Japan Tsunami of 2011
PICES SPECIAL PUBLICATION 6 The Effects of Marine Debris Caused by the Great Japan Tsunami of 2011 Editors: Cathryn Clarke Murray, Thomas W. Therriault, Hideaki Maki, and Nancy Wallace Authors: Stephen Ambagis, Rebecca Barnard, Alexander Bychkov, Deborah A. Carlton, James T. Carlton, Miguel Castrence, Andrew Chang, John W. Chapman, Anne Chung, Kristine Davidson, Ruth DiMaria, Jonathan B. Geller, Reva Gillman, Jan Hafner, Gayle I. Hansen, Takeaki Hanyuda, Stacey Havard, Hirofumi Hinata, Vanessa Hodes, Atsuhiko Isobe, Shin’ichiro Kako, Masafumi Kamachi, Tomoya Kataoka, Hisatsugu Kato, Hiroshi Kawai, Erica Keppel, Kristen Larson, Lauran Liggan, Sandra Lindstrom, Sherry Lippiatt, Katrina Lohan, Amy MacFadyen, Hideaki Maki, Michelle Marraffini, Nikolai Maximenko, Megan I. McCuller, Amber Meadows, Jessica A. Miller, Kirsten Moy, Cathryn Clarke Murray, Brian Neilson, Jocelyn C. Nelson, Katherine Newcomer, Michio Otani, Gregory M. Ruiz, Danielle Scriven, Brian P. Steves, Thomas W. Therriault, Brianna Tracy, Nancy C. Treneman, Nancy Wallace, and Taichi Yonezawa. Technical Editor: Rosalie Rutka Please cite this publication as: The views expressed in this volume are those of the participating scientists. Contributions were edited for Clarke Murray, C., Therriault, T.W., Maki, H., and Wallace, N. brevity, relevance, language, and style and any errors that [Eds.] 2019. The Effects of Marine Debris Caused by the were introduced were done so inadvertently. Great Japan Tsunami of 2011, PICES Special Publication 6, 278 pp. Published by: Project Designer: North Pacific Marine Science Organization (PICES) Lori Waters, Waters Biomedical Communications c/o Institute of Ocean Sciences Victoria, BC, Canada P.O. Box 6000, Sidney, BC, Canada V8L 4B2 Feedback: www.pices.int Comments on this volume are welcome and can be sent This publication is based on a report submitted to the via email to: [email protected] Ministry of the Environment, Government of Japan, in June 2017. -

Transgenic Banana Expressing Pflp Gene Confers Enhanced Resistance
Transgenic Res (2012) 21:855–865 DOI 10.1007/s11248-011-9574-y ORIGINAL PAPER Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease B. Namukwaya • L. Tripathi • J. N. Tripathi • G. Arinaitwe • S. B. Mukasa • W. K. Tushemereirwe Received: 30 June 2011 / Accepted: 11 November 2011 / Published online: 19 November 2011 Ó Springer Science+Business Media B.V. 2011 Abstract Banana Xanthomonas wilt (BXW), caused transgenic lines were characterized by molecular by Xanthomonas campestris pv. musacearum, is one analysis. After challenge with X. campestris pv. of the most important diseases of banana (Musa sp.) musacearum transgenic lines showed high resistance. and currently considered as the biggest threat to About 67% of transgenic lines evaluated were com- banana production in Great Lakes region of East and pletely resistant to BXW. These transgenic lines did Central Africa. The pathogen is highly contagious and not show any disease symptoms after artificial inoc- its spread has endangered the livelihood of millions of ulation of in vitro plants under laboratory conditions as farmers who rely on banana for food and income. The well as potted plants in the screen-house, whereas non- development of disease resistant banana cultivars transgenic control plants showed severe symptoms remains a high priority since farmers are reluctant to resulting in complete wilting. This study confirms that employ labor-intensive disease control measures and expression of the Pflp gene in banana results in there is no host plant resistance among banana enhanced resistance to BXW. This transgenic tech- cultivars. In this study, we demonstrate that BXW nology can provide a timely solution to the BXW can be efficiently controlled using transgenic technol- pandemic. -

ILLEGAL FISHING Which Fish Species Are at Highest Risk from Illegal and Unreported Fishing?
ILLEGAL FISHING Which fish species are at highest risk from illegal and unreported fishing? October 2015 CONTENTS EXECUTIVE SUMMARY 3 INTRODUCTION 4 METHODOLOGY 5 OVERALL FINDINGS 9 NOTES ON ESTIMATES OF IUU FISHING 13 Tunas 13 Sharks 14 The Mediterranean 14 US Imports 15 CONCLUSION 16 CITATIONS 17 OCEAN BASIN PROFILES APPENDIX 1: IUU Estimates for Species Groups and Ocean Regions APPENDIX 2: Estimates of IUU Risk for FAO Assessed Stocks APPENDIX 3: FAO Ocean Area Boundary Descriptions APPENDIX 4: 2014 U.S. Edible Imports of Wild-Caught Products APPENDIX 5: Overexploited Stocks Categorized as High Risk – U.S. Imported Products Possibly Derived from Stocks EXECUTIVE SUMMARY New analysis by World Wildlife Fund (WWF) finds that over 85 percent of global fish stocks can be considered at significant risk of Illegal, Unreported, and Unregulated (IUU) fishing. This evaluation is based on the most recent comprehensive estimates of IUU fishing and includes the worlds’ major commercial stocks or species groups, such as all those that are regularly assessed by the United Nations Food and Agriculture Organization (FAO). Based on WWF’s findings, the majority of the stocks, 54 percent, are categorized as at high risk of IUU, with an additional 32 perent judged to be at moderate risk. Of the 567 stocks that were assessed, the findings show that 485 stocks fall into these two categories. More than half of the world’s most overexploited stocks are at the highest risk of IUU fishing. Examining IUU risk by location, the WWF analysis shows that in more than one-third of the world’s ocean basins as designated by the FAO, all of these stocks were at high or moderate risk of IUU fishing. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -
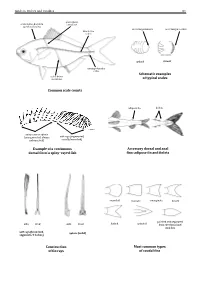
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -

Impact of Banana Xanthomonas Wilt on Farmers' Livelihoods in Kagera
Vol. 9(7), pp. 279-286, July 2015 DOI: 10.5897/AJPS2015.1292 Article Number: 19632ED54360 ISSN 1996-0824 African Journal of Plant Science Copyright © 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/AJPS Full Length Research Paper Adverse impact of Banana Xanthomonas Wilt on farmers’ livelihoods in Eastern and Central Africa Jackson Nkuba1*, William Tinzaara2, Gertrude Night3, Nicholas Niko4, Wellington Jogo2, Innocent Ndyetabula1, Leornard Mukandala1, Privat Ndayihazamaso4, Celestin Niyongere4, Svetlana Gaidashova3, Ivan Rwomushana5, Fina Opio5 and Eldad Karamura2 1Maruku-Agricultural Research and Development Institute, P.O. Box 127, Bukoba, Tanzania. 2Bioversity International, P.O. Box 24384, Kampala, Uganda. 3Rwanda Agriculture Board (RAB), Rwanda. 4Institut des Sciences Agronomiques du Burundi (ISABU), Burundi. 5Association for Strengthening Agricultural Research in Eastern and Central Africa, P.O. 765, Entebbe, Uganda. Received 9 March, 2015; Accepted 5 May, 2015 Banana is a key crop in the livelihoods of many people in the Great Lakes region of East and Central Africa. For more than a decade now, the crop has been threatened by Banana Xanthomonas Wilt (BXW) which has spread throughout the region but at different rates. The disease attacks all banana cultivars and can cause up to 100% yield losses at farm level if effective control measures are not put in place. However, limited information on impact of BXW at regional level is available to guide interventions. Thus, this study assessed the impact of BXW on farmers’ livelihoods in Kagera basin of Tanzania, Burundi and Rwanda. A total of 436 households (Tanzania 120, Burundi 208 and Rwanda 108) mostly from major banana-producing and BXW-affected districts were sampled and interviewed in a household survey. -

Foxr1 Is a Novel Maternal-Effect Gene in Fish That Is Required for Early Embryonic Success Caroline Cheung, Amélie Patinote, Yann Guiguen, Julien Bobe
Foxr1 is a novel maternal-effect gene in fish that is required for early embryonic success Caroline Cheung, Amélie Patinote, Yann Guiguen, Julien Bobe To cite this version: Caroline Cheung, Amélie Patinote, Yann Guiguen, Julien Bobe. Foxr1 is a novel maternal- effect gene in fish that is required for early embryonic success. PeerJ, PeerJ, 2018, 6,pp.1-20. 10.7717/peerj.5534. hal-01867990 HAL Id: hal-01867990 https://hal.archives-ouvertes.fr/hal-01867990 Submitted on 4 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License foxr1 is a novel maternal-effect gene in fish that is required for early embryonic success Caroline T. Cheung, Amélie Patinote, Yann Guiguen and Julien Bobe LPGP, UR1037 Fish Physiology and Genomics, INRA, Rennes, France ABSTRACT The family of forkhead box (Fox) transcription factors regulates gonadogenesis and embryogenesis, but the role of foxr1 in reproduction is unknown. Evolutionary history of foxr1 in vertebrates was examined and the gene was found to exist in most vertebrates, including mammals, ray-finned fish, amphibians, and sauropsids. By quantitative PCR and RNA-seq, we found that foxr1 had an ovarian-specific expression in zebrafish, a common feature of maternal-effect genes. -

Banana Xanthomonas Wilt: a Review of the Disease, Management Strategies and Future Research Directions
African Journal of Biotechnology Vol. 6 (8), pp. 953-962, 16 April 2007 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2007 Academic Journals Review Banana Xanthomonas wilt: a review of the disease, management strategies and future research directions Moses Biruma2, Michael Pillay1,2*, Leena Tripathi2, Guy Blomme3, Steffen Abele2, Maina Mwangi2, Ranajit Bandyopadhyay4, Perez Muchunguzi2, Sadik Kassim2, Moses Nyine2 Laban Turyagyenda2 and Simon Eden-Green5 1Vaal University of Technology, Private Bag X021, Vanderbijlpark 1900, South Africa. 2International Institute of Tropical Agriculture (IITA), P. O. Box 7878, Kampala, Uganda 3International Network for the Improvement of Banana and Plantain (INIBAP) P. O. Box 24384 Kampala, Uganda 4International Institute of Tropical Agriculture, Ibadan, Nigeria 5EG Consulting, 470 Lunsford Lane, Larkfield, Kent ME20 6JA, United Kingdom. Accepted 1 March, 2007 Banana production in Eastern Africa is threatened by the presence of a new devastating bacterial disease caused by Xanthomonas vasicola pv. musacearum (formerly Xanthomonas campestris pv. musacearum). The disease has been identified in Uganda, Eastern Democratic Republic of Congo, Rwanda and Tanzania. Disease symptoms include wilting and yellowing of leaves, excretion of a yel- lowish bacterial ooze, premature ripening of the bunch, rotting of fruit and internal yellow discoloration of the vascular bundles. Plants are infected either by insects through the inflorescence or by soil-borne bacterial inoculum through the lower parts of the plant. Short- and long-distance transmission of the disease mainly occurs via contaminated tools and insects, though other organisms such as birds may also be involved. Although no banana cultivar with resistance to the disease has been identified as yet, it appears that certain cultivars have mechanisms to ‘escape’ the disease. -

Fao Species Catalogue
FAO Fisheries Synopsis No. 125, Volume 2 FIR/S125 Vol. 2 FAO SPECIES CATALOGUE VOL. 2 SCOMBRIDS OF THE WORLD AN ANNOTATED AND ILLUSTRATED CATALOGUE OF TUNAS, MACKERELS, BONITOS, AND RELATED SPECIES KNOWN TO DATE UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO Fisheries Synopsis No. 125, Volume 2 FIR/S125 Vol. 2 FAO SPECIES CATALOGUE VOL. 2 SCOMBRIDS OF THE WORLD An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date prepared by Bruce B. Collette and Cornelia E. Nauen NOAA, NMFS Marine Resources Service Systematics Laboratory Fishery Resources and Environment Division National Museum of Natural History FAO Fisheries Department Washington, D.C. 20560, USA 00100 Rome, Italy UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome 1983 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-42 ISBN 92-5-101381-0 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome Italy. -

Diagnostic and Management Guide Xanthomonas Wilt of Bananas
Xanthomonas Wilt of Bananas in East and Central Africa Diagnostic and Management Guide E. B. Karamura, F. L. Turyagyenda, W. Tinzaara, G. Blomme, F. Ssekiwoko, S. Eden–Green, A. Molina & R. Markham Bioversity International Rome, Italy Bioversity Kampala, Uganda Bioversity International is an independent international scientific organization that seeks to improve the well-being of present and future generations of people by enhancing conservation and the deployment of agricultural biodiversity on farms and in forests. It is one of 15 centres supported by the Consultative Group on International Agricultural Research (CGIAR), an association of public and private members who support efforts to mobilize cutting-edge science to reduce hunger and poverty, improve human nutrition and health, and protect the environment. Bioversity has its headquarters in Maccarese, near Rome, Italy, with offices in more than 20 other countries worldwide. The Institute operates through four Programmemes: Diversity for Livelihoods, Understanding and Managing Biodiversity, Global Partnerships, and Commodities for Livelihoods. The international status of Bioversity is conferred under an Establishment Agreement which, by January 2008, had been signed by the Governments of Algeria, Australia, Belgium, Benin, Bolivia, Brazil, Burkina Faso, Cameroon, Chile, China, Congo, Costa Rica, COte d’lvoire, Cyprus, Czech Republic, Denmark, Ecuador, Egypt, Ethiopia, Ghana, Greece, Guinea, Hungary, India, Indonesia, Iran, Israel, Italy, Jordan, Kenya, Malaysia, Mali, Mauritania,