Optimal Foraging and the Role of Compensatory Growth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Building Trophic Specializations That Result in Substantial Niche
Journal of Anatomy J. Anat. (2017) doi: 10.1111/joa.12742 Building trophic specializations that result in substantial niche partitioning within a young adaptive radiation Luz Patricia Hernandez,1 Dominique Adriaens,2 Christopher H. Martin,3 Peter C. Wainwright,4 Bert Masschaele5 and Manuel Dierick5 1Department of Biological Sciences, The George Washington University, Washington, DC, USA 2Evolutionary Morphology of Vertebrates, Ghent University, Ghent, Belgium 3Department of Biology, University of North Carolina, Chapel Hill, Chapel Hill, NC, USA 4Department of Evolution & Ecology, University of California, Davis, Davis, CA, USA 5Department of Subatomic and Radiation Physics, Ghent University, Ghent, Belgium Abstract Dietary partitioning often accompanies the increased morphological diversity seen during adaptive radiations within aquatic systems. While such niche partitioning would be expected in older radiations, it is unclear how significant morphological divergence occurs within a shorter time period. Here we show how differential growth in key elements of the feeding mechanism can bring about pronounced functional differences among closely related species. An incredibly young adaptive radiation of three Cyprinodon species residing within hypersaline lakes in San Salvador Island, Bahamas, has recently been described. Characterized by distinct head shapes, gut content analyses revealed three discrete feeding modes in these species: basal detritivory as well as derived durophagy and lepidophagy (scale-feeding). We dissected, cleared and stained, and micro-CT scanned species to assess functionally relevant differences in craniofacial musculoskeletal elements. The widespread feeding mode previously described for cyprinodontiforms, in which the force of the bite may be secondary to the requisite dexterity needed to pick at food items, is modified within both the scale specialist and the durophagous species. -

Reptiles As Principal Prey? Adaptations for Durophagy and Prey Selection by Jaguar (Panthera Onca) Everton B.P
JOURNAL OF NATURAL HISTORY, 2016 http://dx.doi.org/10.1080/00222933.2016.1180717 Reptiles as principal prey? Adaptations for durophagy and prey selection by jaguar (Panthera onca) Everton B.P. Mirandaa,b, Jorge F.S. de Menezesc,d and Marcelo L. Rheingantzc aLaboratório de Herpetologia, Universidade Federal de Mato Grosso, Cuiabá, Brazil; bPrograma de Pós-graduação em Ecologia e Conservação da Biodiversidade, Universidade Federal de Mato Grosso, Cuiabá, Brasil; cLaboratório de Ecologia e Conservação de Populações, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil; dMitrani Department of Desert Ecology, Ben Gurion University of the Negev, Beersheba, Israel ABSTRACT ARTICLE HISTORY We examined the evidence supporting the hypothesis that jaguars Received 16 February 2015 (Panthera onca) have morphological and behavioural adaptations Accepted 21 January 2016 ’ to facilitate reptile predation. Jaguars head and bite features show KEYWORDS adaptations to durophagy (consumption of hard-integumented Dangerous prey; prey) that are very unusual within the genus Panthera. These saurophagy; Tayassuidae; include: thick canines, well-developed head muscles and a fatal Xenarthra bite directed to braincase or nape. These characteristics have been previously considered an adaptation for the consumption of rep- tilian prey, whose thick integument poses a challenge to preda- tion. Although causation of any trait as result of natural selection is hard to demonstrate with ecological evidence, its consequences can be suggested and predictions made. Here, through a review of the literature on jaguar predatory habits, we tallied the evidence for saurophagy against environmental characteristics correlated with jaguar predation on reptiles. We offer a new explanation for the presence of those traits, based on the selection patterns, prey abundances and main predation habits over the geographic range of the jaguar. -

Ontogeny of Jaw Mechanics and Stiffness in Mollusk‐Feeding
Received: 18 December 2018 Revised: 15 March 2019 Accepted: 16 March 2019 DOI: 10.1002/jmor.20984 RESEARCH ARTICLE Killing them softly: Ontogeny of jaw mechanics and stiffness in mollusk-feeding freshwater stingrays Kelsi M. Rutledge1,2 | Adam P. Summers1 | Matthew A. Kolmann3,4,1 1Department of Biology, Friday Harbor Laboratories, University of Washington, Friday Abstract Harbor, Washington Durophagous predators consume hard-shelled prey such as bivalves, gastropods, and 2 Department of Ecology and Evolutionary large crustaceans, typically by crushing the mineralized exoskeleton. This is costly Biology, University of California, Los Angeles, California from the point of view of the bite forces involved, handling times, and the stresses 3Department of Biological Sciences, George inflicted on the predator's skeleton. It is not uncommon for durophagous taxa to dis- Washington University, Washington, D.C. play an ontogenetic shift from softer to harder prey items, implying that it is relatively 4Department of Ichthyology, Royal Ontario Museum, Toronto, Ontario difficult for smaller animals to consume shelled prey. Batoid fishes (rays, skates, sawfishes, and guitarfishes) have independently evolved durophagy multiple times, Correspondence Kelsi M. Rutledge, Department of Ecology and despite the challenges associated with crushing prey harder than their own cartilagi- Evolutionary Biology, University of California nous skeleton. Potamotrygon leopoldi is a durophagous freshwater ray endemic to the Los Angeles, Los Angeles, CA 90095. Email: [email protected]; Xingu River in Brazil, with a jaw morphology superficially similar to its distant [email protected] durophagous marine relatives, eagle rays (e.g., Aetomylaeus, Aetobatus). We used sec- Present address ond moment of area as a proxy for the ability to resist bending and analyzed the Kelsi M. -

Variable Changes in Cranial Structures Underlie Transitions to Durophagy in Moray Eels
http://www.jstor.org/stable/10.1086/675810 . Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press, The American Society of Naturalists, The University of Chicago are collaborating with JSTOR to digitize, preserve and extend access to The American Naturalist. http://www.jstor.org This content downloaded from 128.120.194.194 on Fri, 2 May 2014 08:16:53 AM All use subject to JSTOR Terms and Conditions vol. 183, no. 6 the american naturalist june 2014 E-Article Imperfect Morphological Convergence: Variable Changes in Cranial Structures Underlie Transitions to Durophagy in Moray Eels David C. Collar,1,*JoshuaS.Reece,2 Michael E. Alfaro,3 Peter C. Wainwright,1 and Rita S. Mehta4 1. Department of Evolution and Ecology, University of California, Davis, California 95616; 2. Department of Biology, Valdosta State University, Valdosta, Georgia 31698; 3. Department of Ecology and Evolutionary Biology, University of California, Los Angeles, California 90095; 4. Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, California 95060 Submitted October 8, 2013; Accepted December 27, 2013; Electronically published April 24, 2014 Online enhancement: appendixes. Dryad data: http://dx.doi.org/10.5061/dryad.h9r37. -

WILDLIFE JOURNAL SINGITA PAMUSHANA, ZIMBABWE for the Month of March, Two Thousand and Nineteen
WILDLIFE JOURNAL SINGITA PAMUSHANA, ZIMBABWE For the month of March, Two Thousand and Nineteen Temperature Rainfall Recorded Sunrise & Sunset Average minimum: 21˚C (69,8˚F) For the month: 69 mm Sunrise: 06:00 Minimum recorded: 18,8˚C (65,8˚F) For the year to date: 238 mm Sunset: 17:55 Average maximum: 31,1˚C (87,9˚F) Maximum recorded: 36,6˚C (97,8˚F) We can report that the final phase of lodge renovation at Singita Pamushana is going well, and us field guides have been busying ourselves with all kinds of projects and taking our annual leave while there are no guests visiting. Fortunately, we weren’t really affected by the cyclone that hit Mozambique in March, however the Malilangwe Trust and our staff donated goods to those most affected. We have had some good rain and the landscape is looking lush. Here’s a Sightings Snapshot for March: Lions • There’s been a very interesting development in that the River Pride has taken over part of the Southern Pride’s territory. We are now seeing the River Pride in the southern area and it’ll be interesting to see how this plays out. • Great news is that the three lion cubs that seemed to have disappeared a while ago have reappeared, and are in good health. Leopards • We’ve heard a lot of leopard calling at night, so there is much activity even though we are not on drive, and in the last two days we have had three leopard sightings! Cheetah • Cheetahs were spotted on staff drives. -

SPECIAL PUBLICATION 6 the Effects of Marine Debris Caused by the Great Japan Tsunami of 2011
PICES SPECIAL PUBLICATION 6 The Effects of Marine Debris Caused by the Great Japan Tsunami of 2011 Editors: Cathryn Clarke Murray, Thomas W. Therriault, Hideaki Maki, and Nancy Wallace Authors: Stephen Ambagis, Rebecca Barnard, Alexander Bychkov, Deborah A. Carlton, James T. Carlton, Miguel Castrence, Andrew Chang, John W. Chapman, Anne Chung, Kristine Davidson, Ruth DiMaria, Jonathan B. Geller, Reva Gillman, Jan Hafner, Gayle I. Hansen, Takeaki Hanyuda, Stacey Havard, Hirofumi Hinata, Vanessa Hodes, Atsuhiko Isobe, Shin’ichiro Kako, Masafumi Kamachi, Tomoya Kataoka, Hisatsugu Kato, Hiroshi Kawai, Erica Keppel, Kristen Larson, Lauran Liggan, Sandra Lindstrom, Sherry Lippiatt, Katrina Lohan, Amy MacFadyen, Hideaki Maki, Michelle Marraffini, Nikolai Maximenko, Megan I. McCuller, Amber Meadows, Jessica A. Miller, Kirsten Moy, Cathryn Clarke Murray, Brian Neilson, Jocelyn C. Nelson, Katherine Newcomer, Michio Otani, Gregory M. Ruiz, Danielle Scriven, Brian P. Steves, Thomas W. Therriault, Brianna Tracy, Nancy C. Treneman, Nancy Wallace, and Taichi Yonezawa. Technical Editor: Rosalie Rutka Please cite this publication as: The views expressed in this volume are those of the participating scientists. Contributions were edited for Clarke Murray, C., Therriault, T.W., Maki, H., and Wallace, N. brevity, relevance, language, and style and any errors that [Eds.] 2019. The Effects of Marine Debris Caused by the were introduced were done so inadvertently. Great Japan Tsunami of 2011, PICES Special Publication 6, 278 pp. Published by: Project Designer: North Pacific Marine Science Organization (PICES) Lori Waters, Waters Biomedical Communications c/o Institute of Ocean Sciences Victoria, BC, Canada P.O. Box 6000, Sidney, BC, Canada V8L 4B2 Feedback: www.pices.int Comments on this volume are welcome and can be sent This publication is based on a report submitted to the via email to: [email protected] Ministry of the Environment, Government of Japan, in June 2017. -

ILLEGAL FISHING Which Fish Species Are at Highest Risk from Illegal and Unreported Fishing?
ILLEGAL FISHING Which fish species are at highest risk from illegal and unreported fishing? October 2015 CONTENTS EXECUTIVE SUMMARY 3 INTRODUCTION 4 METHODOLOGY 5 OVERALL FINDINGS 9 NOTES ON ESTIMATES OF IUU FISHING 13 Tunas 13 Sharks 14 The Mediterranean 14 US Imports 15 CONCLUSION 16 CITATIONS 17 OCEAN BASIN PROFILES APPENDIX 1: IUU Estimates for Species Groups and Ocean Regions APPENDIX 2: Estimates of IUU Risk for FAO Assessed Stocks APPENDIX 3: FAO Ocean Area Boundary Descriptions APPENDIX 4: 2014 U.S. Edible Imports of Wild-Caught Products APPENDIX 5: Overexploited Stocks Categorized as High Risk – U.S. Imported Products Possibly Derived from Stocks EXECUTIVE SUMMARY New analysis by World Wildlife Fund (WWF) finds that over 85 percent of global fish stocks can be considered at significant risk of Illegal, Unreported, and Unregulated (IUU) fishing. This evaluation is based on the most recent comprehensive estimates of IUU fishing and includes the worlds’ major commercial stocks or species groups, such as all those that are regularly assessed by the United Nations Food and Agriculture Organization (FAO). Based on WWF’s findings, the majority of the stocks, 54 percent, are categorized as at high risk of IUU, with an additional 32 perent judged to be at moderate risk. Of the 567 stocks that were assessed, the findings show that 485 stocks fall into these two categories. More than half of the world’s most overexploited stocks are at the highest risk of IUU fishing. Examining IUU risk by location, the WWF analysis shows that in more than one-third of the world’s ocean basins as designated by the FAO, all of these stocks were at high or moderate risk of IUU fishing. -

(Crustacea) As Predators on Mollusca Through Geologic Time
PALAIOS, 2010, v. 25, p. 167–182 Research Article DOI: 10.2110/palo.2009.p09-054r THE DECAPODA (CRUSTACEA) AS PREDATORS ON MOLLUSCA THROUGH GEOLOGIC TIME CARRIE E. SCHWEITZER1* and RODNEY M. FELDMANN 2 1Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. NW, North Canton, Ohio 44720, USA; 2Department of Geology, Kent State University, Kent, Ohio 44242, USA e-mail: [email protected] ABSTRACT decapods and lineages known to have modern congeners that are durophagous. The relationship between predator and prey is a persistent theme in There are five major means by which decapods crush shells or eat marine paleontology. Herein we focus on the decapod Crustacea, the shelled prey that, as they require specialization of the appendages (see shrimps, lobsters, and crabs, and their role as predators on the Mollusca Lau, 1987 on extant forms), might be recorded in the body fossil record. through geologic time. Five major means by which decapods crush shells There are also several types of feeding that may only chip the shells or or eat shelled prey might be recorded in the body-fossil record, as they simply pry them open, which are often not apparent in the fossil record. require specialization of the appendages. These include use of (1) All types of feeding on shelled organisms must affect evolution of the heterochelous first pereiopods, (2) molariform teeth on the fingers of shelled organism, based on recent studies that illustrate how predation the chelae, (3) a curved proximal tooth on the movable finger of the chela, takes precedence over other aspects of the environment in driving (4) calcified mandibles, and (5) flattened pereiopods (walking legs). -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -
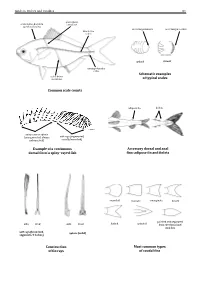
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -

A Dental Microwear Texture Analysis of the Mio−Pliocene Hyaenids from Langebaanweg, South Africa
A dental microwear texture analysis of the Mio−Pliocene hyaenids from Langebaanweg, South Africa DEANO D. STYNDER, PETER S. UNGAR, JESSICA R. SCOTT, and BLAINE W. SCHUBERT Stynder, D.D., Ungar, P.S., Scott, J.R., and Schubert, B.W. 2012. A dental microwear texture analysis of the Mio−Plio− cene hyaenids from Langebaanweg, South Africa. Acta Palaeontologica Polonica 57 (3): 485–496. Hyaenids reached their peak diversity during the Mio−Pliocene, when an array of carnivorous species emerged alongside dwindling civet−like and mongoose−like insectivorous/omnivorous taxa. Significantly, bone−cracking morphological ad− aptations were poorly developed in these newly−emerged species. This, their general canid−like morphology, and the ab− sence/rarity of canids in Eurasia and Africa at the time, has led researchers to hypothesise that these carnivorous Mio−Pliocene hyaenas were ecological vicars to modern canids. To shed further light on their diets and foraging strate− gies, we examine and compare the dental microwear textures of Hyaenictitherium namaquensis, Ikelohyaena abronia, Chasmaporthetes australis, and Hyaenictis hendeyi from the South African Mio−Pliocene site of Langebaanweg with those of the extant feliforms Crocuta crocuta, Acinonyx jubatus, and Panthera leo (caniforms are not included because homologous wear facets are not directly comparable between the suborders). Sample sizes for individual fossil species are small, which limits confidence in assessments of variation between the extinct taxa; however, these Mio−Pliocene hyaenas exhibit surface complexity and textural fill volume values that are considerably lower than those exhibited by the living hyaena, Crocuta crocuta. Dental microwear texture analysis thus supports interpretations of craniodental evidence suggesting low bone consumption in carnivorous Mio−Pliocene hyaenas.