The Inactivating Potassium Currents of Hair Cells Isolated from the Crista Ampullaris of the Frog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Facilitation of Rabbit ב1B Calcium Channels: Involvement of Endogenous Gגד Subunits
Keywords: Calcium channel, G protein, Facilitation 7535 Journal of Physiology (1998), 509.1, pp.15—27 15 Facilitation of rabbit á1B calcium channels: involvement of endogenous Gâã subunits Gary J. Stephens, Nicola L. Brice, Nicholas S. Berrow and Annette C. Dolphin Department of Pharmacology, University College London and Royal Free Hospital School of Medicine, London WC1E 6BT, UK (Received 30 October 1997; accepted after revision 26 January 1998) 1. The á1B (N-type) calcium channel shows strong G protein modulation in the presence of G protein activators or Gâã subunits. Using transient expression in COS_7 cells of á1B together with the accessory subunits áµ—ä and â2a,wehaveexaminedtheroleofendogenous Gâã subunits in the tonic modulation of á1B, and compared this with modulation by exogenously expressed Gâã subunits. 2. Prepulse facilitation of control á1BÏáµ—äÏâ2a currents was always observed. This suggests the existenceoftonicmodulationofá1B subunits. To determine whether endogenous Gâã is involved in the facilitation observed in control conditions, the âARK1 Gâã-binding domain (amino acids 495—689) was overexpressed, in order to bind free Gâã subunits. The extent of control prepulse-induced facilitation was significantly reduced, both in terms of current amplitude and the rate of current activation. In agreement with this, GDPâS also reduced the control facilitation. 3. Co-expression of the GâÔãµ subunit, together with the á1BÏáµ—äÏâ2a calcium channel combination, resulted in a marked degree of depolarizing prepulse-reversible inhibition of the whole-cell ICa or IBa. Both slowing of current activation and inhibition of the maximum current amplitude were observed, accompanied by a depolarizing shift in the mid-point of the voltage dependence of activation. -
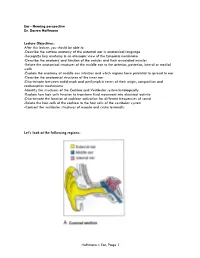
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

An Unexpected Role for Brain-Type Sodium Channels in Coupling of Cell Surface Depolarization to Contraction in the Heart
An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart Sebastian K. G. Maier*, Ruth E. Westenbroek*, Kenneth A. Schenkman†, Eric O. Feigl‡, Todd Scheuer*, and William A. Catterall*§ Departments of *Pharmacology, †Pediatrics, and ‡Physiology and Biophysics, University of Washington, Seattle, WA 98195 Contributed by William A. Catterall, December 27, 2001 ␣ Voltage-gated sodium channels composed of pore-forming and ventricular myocytes compared with Nav1.5, but they contribute auxiliary  subunits are responsible for the rising phase of the significantly to coupling of cell surface depolarization to con- action potential in cardiac muscle, but the functional roles of traction because of their location in the transverse tubules. Our distinct sodium channel subtypes have not been clearly defined. results provide the first evidence, to our knowledge, for a unique Immunocytochemical studies show that the principal cardiac pore- functional role of TTX-sensitive brain-type sodium channels in forming ␣ subunit isoform Nav1.5 is preferentially localized in the heart. intercalated disks, whereas the brain ␣ subunit isoforms Nav1.1, Nav1.3, and Nav1.6 are localized in the transverse tubules. Sodium Methods currents due to the highly tetrodotoxin (TTX)-sensitive brain iso- All procedures were approved by the Institutional Animal Care forms in the transverse tubules are small and are detectable only and Use Committee of the University of Washington. after activation with  scorpion toxin. Nevertheless, they play an important role in coupling depolarization of the cell surface mem- Cell Isolation. Ventricular myocytes were isolated from adult male brane to contraction, because low TTX concentrations reduce left (8–10 weeks) wild-type B6129F1 mice, as described (14). -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

The Role of S4 Charges in Voltage-Dependent
ARTICLE The Role of S4 Charges in Voltage-dependent and Voltage-independent KCNQ1 Potassium Channel Complexes Gianina Panaghie and Geoffrey W. Abbott Greenberg Division of Cardiology, Department of Medicine and Department of Pharmacology, Cornell University, Weill Medical College, New York, NY 10021 Voltage-gated potassium (Kv) channels extend their functional repertoire by coassembling with MinK-related peptides (MiRPs). MinK slows the activation of channels formed with KCNQ1 α subunits to generate the voltage- dependent IKs channel in human heart; MiRP1 and MiRP2 remove the voltage dependence of KCNQ1 to generate potassium “leak” currents in gastrointestinal epithelia. Other Kv α subunits interact with MiRP1 and MiRP2 but without loss of voltage dependence; the mechanism for this disparity is unknown. Here, sequence alignments re- vealed that the voltage-sensing S4 domain of KCNQ1 bears lower net charge (+3) than that of any other eukaryotic voltage-gated ion channel. We therefore examined the role of KCNQ1 S4 charges in channel activation using alanine-scanning mutagenesis and two-electrode voltage clamp. Alanine replacement of R231, at the N-terminal side of S4, produced constitutive activation in homomeric KCNQ1 channels, a phenomenon not observed with previous single amino acid substitutions in S4 of other channels. Homomeric KCNQ4 channels were also made constitutively active by mutagenesis to mimic the S4 charge balance of R231A-KCNQ1. Loss of single S4 charges at positions R231 or R237 produced constitutively active MinK-KCNQ1 channels and increased the constitutively active component of MiRP2-KCNQ1 currents. Charge addition to the CO2H-terminal half of S4 eliminated constitu- tive activation in MiRP2-KCNQ1 channels, whereas removal of homologous charges from KCNQ4 S4 produced constitutively active MiRP2-KCNQ4 channels. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

ACTX-Hv1c, Isolated from the Venom of the Blue Mountains
S.J. Gunning et al. Janus-faced atracotoxins block KCa channels The Janus-faced atracotoxins are specific blockers of invertebrate KCa channels Simon J. Gunning1, Francesco J. Maggio2,4, Monique J. Windley1, Stella M. Valenzuela1, Glenn F. King3 and Graham M. Nicholson1 1 Neurotoxin Research Group, Department of Medical & Molecular Biosciences, University of Technology, Sydney, Broadway, NSW 2007, Australia 2 Department of Molecular, Microbial & Structural Biology, University of Connecticut School of Medicine, Farmington, Connecticut 06032, USA and 3 Division of Chemical and Structural Biology, Institute for Molecular Bioscience, University of Queensland, Brisbane QLD 4072, Australia 4 Current affiliation: Bristol-Myers Squibb, 6000 Thompson Road, Syracuse NY 13057, USA Subdivision: Molecular Neurobiology Abbreviations: ACTX, atracotoxin; 4-AP, 4-aminopyridine; BKCa channel, 2+ + 2+ large-conductance Ca -activated K channel; CaV channel, voltage-activated Ca channel; ChTx, charybdotoxin; DRG, dorsal root ganglia; DUM, dorsal unpaired median; EGTA, ethylene glycol-bis(b-aminoethyl ether)-N,N,N’N’-tetraacetic acid; IbTx, + iberiotoxin; J-ACTX, Janus-faced atracotoxin; KA channel, transient ‘A-type’ K channel; 2+ + + KCa channel, Ca -activated K channel; KDR channel, delayed-rectifier K channel; KV + + channel, voltage-activated K channel; NaV channel, voltage-activated Na channel; Slo, Slowpoke; TEA, tetraethylammonium; TTX, tetrodotoxin. RUNNING TITLE: Janus-faced atracotoxins block KCa channels ADDRESS CORRESPONDENCE TO: Graham M. Nicholson, Department of Medical & Molecular Biosciences, University of Technology, Sydney, PO Box 123, Broadway NSW 2007, Australia. Tel: +61 2 9514-2230 Fax: +61 2 9514-2228; E-mail: [email protected] 1 S.J. Gunning et al. Janus-faced atracotoxins block KCa channels ABSTRACT The Janus-faced atracotoxins are a unique family of excitatory peptide toxins that contain a rare vicinal disulfide bridge. -

Uhm Phd 9118021 R.Pdf
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. U·M·I University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road. Ann Arbor. M148106·1346 USA 313/761-4700 800/521-0600 Order Number 9118021 Sodium channel activation mechanisms: Insights from deuterium oxide and ddta-9-tetrahydrocannabinol substitution Alicata, Daniel Andrew, Ph.D. -

Phenytoin Inhibits the Persistent Sodium Current in Neocortical Neurons by Modifying Its Inactivation Properties
Phenytoin Inhibits the Persistent Sodium Current in Neocortical Neurons by Modifying Its Inactivation Properties Elisa Colombo1, Silvana Franceschetti1, Giuliano Avanzini1, Massimo Mantegazza1,2* 1 Department of Neurophysiopathology – Epilepsy Center, Foundation IRCCS Neurological Institute C. Besta, Milan, Italy, 2 Institut de Pharmacologie Mole´culaire et Cellulaire (IPMC), CNRS UMR7275 and University of Nice-Sophia Antipolis, Valbonne, France Abstract + The persistent Na current (INaP) is important for neuronal functions and can play a role in several pathologies, although it is + small compared to the transient Na current (INaT). Notably, INaP is not a real persistent current because it undergoes inactivation with kinetics in the order of tens of seconds, but this property has often been overlooked. Na+ channel blockers, drugs used for treating epilepsy and other diseases, can inhibit INaP, but the mechanism of this action and the conditions in which INaP can be actually inhibited have not been completely clarified yet. We evaluated the action of phenytoin (PHT), a + prototype anti-epileptic Na channel blocker, on INaP inactivation in pyramidal neurons of rat sensorimotor cortical slices at different concentrations, from 5 to 100 mM. PHT did not modify INaP evoked with depolarizing voltage ramps of 50 or 21 21 100 mVs , but decreased INaP evoked by slower voltage ramps (10 mVs ). However, at all of the tested concentrations, PHT decreased INaP evoked by faster ramps when they were preceded by inactivating pre-pulses. Moreover, PHT shifted towards negative potentials the voltage-dependence of INaP inactivation and accelerated its kinetics of development also at depolarized potentials (+40 mV), not consistently with a simple inactivated state stabilizer. -

A Place Principle for Vertigo
Auris Nasus Larynx 35 (2008) 1–10 www.elsevier.com/locate/anl A place principle for vertigo Richard R. Gacek * Department of Otolaryngology, Head and Neck Surgery, University of Massachusetts Medical School, Worcester, MA 01655, USA Received 16 March 2007; accepted 13 April 2007 Available online 24 October 2007 Abstract Objective: To provide a road map of the vestibular labyrinth and its innervation leading to a place principle for different forms of vertigo. Method: The literature describing the anatomy and physiology of the vestibular system was reviewed. Results: Different forms of vertigo may be determined by the type of sense organ, type of ganglion cell and location in the vestibular nerve. Conclusion: Partial lesions (viral) of the vestibular ganglion are manifested as various forms of vertigo. # 2007 Elsevier Ireland Ltd. All rights reserved. Keywords: Vertigo; Vestibular nerve; Pathology Contents 1. Introduction . ............................................................................... 1 2. Sense organ. ............................................................................... 2 3. Ganglion cells ............................................................................... 4 4. Hair cells . ............................................................................... 5 5. Hair cell polarization . ....................................................................... 5 6. Efferent vestibular system ....................................................................... 8 7. A place principle for vertigo . ................................................................. -

Bioinsecticides for the Control of Human Disease Vectors Niraj S Bende B
Bioinsecticides for the control of human disease vectors Niraj S Bende B. Pharm, MRes. Bioinformatics A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2014 Institute for Molecular Bioscience Abstract Many human diseases such as malaria, Chagas disease, chikunguniya and dengue fever are transmitted via insect vectors. Control of human disease vectors is a major worldwide health issue. After decades of persistent use of a limited number of chemical insecticides, vector species have developed resistance to virtually all classes of insecticides. Moreover, considering the hazardous effects of some chemical insecticides to environment and the scarce introduction of new insecticides over the last 20 years, there is an urgent need for the discovery of safe, potent, and eco-friendly bioinsecticides. To this end, the entomopathogenic fungus Metarhizium anisopliae is a promising candidate. For this approach to become viable, however, limitations such as slow onset of death and high cost of currently required spore doses must be addressed. Genetic engineering of Metarhizium to express insecticidal toxins has been shown to increase the potency and decrease the required spore dose. Thus, the primary aim of my thesis was to engineer transgenes encoding highly potent insecticidal spider toxins into Metarhizium in order to enhance its efficacy in controlling vectors of human disease, specifically mosquitoes and triatomine bugs. As a prelude to the genetic engineering studies, I surveyed 14 insecticidal spider venom peptides (ISVPs) in order to compare their potency against key disease vectors (mosquitoes and triatomine bugs) In this thesis, we present the structural and functional analysis of key ISVPs and describe the engineering of Metarhizium strains to express most potent ISVPs. -

Somatic Depolarization Enhances GABA Release in Cerebellar Interneurons Via a Calcium/Protein Kinase C Pathway
5804 • The Journal of Neuroscience, April 13, 2011 • 31(15):5804–5815 Cellular/Molecular Somatic Depolarization Enhances GABA Release in Cerebellar Interneurons via a Calcium/Protein Kinase C Pathway Brice Bouhours, Federico F. Trigo, and Alain Marty Laboratoire de Physiologie Ce´re´brale, Centre National de la Recherche Scientifique and Universite´ Paris Descartes, 75006 Paris, France In cortical and hippocampal neurons, tonic somatic depolarization is partially transmitted to synaptic terminals, where it enhances transmitter release. It is not known to what extent such “analog signaling” applies to other mammalian neurons, and available evidence concerning underlying mechanisms is fragmentary and partially controversial. In this work, we investigate the presence of analog signaling in molecular layer interneurons of the rat cerebellum. GABA release was estimated by measuring autoreceptor currents in single recordings, or postsynaptic currents in paired recordings of synaptically connected neurons. We find with both assays that moderatesubthresholdsomaticdepolarizationresultsinenhancedGABArelease.Inaddition,changesinthecalciumconcentrationwere investigated in the axon compartment using the calcium-sensitive dye OGB-1 (Oregon Green BAPTA-1). After a step somatic depolariza- tion, the axonal calcium concentration and the GABA release probability rise with a common slow time course. However, the amount of calcium entry that is associated to one action potential is not affected. The slow increase in calcium concentration is inhibited by the P/Q calcium channel blocker -agatoxin-IVA. The protein kinase C inhibitor Ro 31-8220 (3-[3-[2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5- dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl carbamimidothioic acid ester mesylate) did not affect the calcium concentration changes butitblockedtheincreaseinGABArelease.EGTAwasaweakblockerofanalogsignaling,implicatingacloseassociationofproteinkinase C to the site of calcium entry.