Unit 13 Eye and Ear.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

Evaluation of the Eyebrow Position After External Müller's Muscle
Journal of Plastic, Reconstructive & Aesthetic Surgery (2019) 72, 662–668 Evaluation of the eyebrow position after external Müller’s muscle tucking: A new technique for ptosis repair a , ∗ b c Kenichi Kokubo , Nobutada Katori , Kengo Hayashi , d e f a Jun Sugawara , Seiko Kou , Akiko Fujii , Shoko Haga , f Jiro Maegawa a Department of Plastic Surgery, Fujisawa Shounandai Hospital. 2345 Takakura, Fujisawa-shi, Kanagawa 251-0802, Japan b Department of Ocular Plastic & Orbital Surgery, Seirei Hamamatsu General Hospital. 2-12-12 Sumiyoshi, Naka-ku, Hamamatsu-shi, Shizuoka 430-8558, Japan c Yokohama Sakuragicho Eye Clinic. 1-200 Hinodecho, Naka-ku Yokohama-shi, Kanagawa 231-0006, Japan d JUN CLINIC, 1402-5 Kitaishidocho, Nagano-shi, Nagano 380-0826, Japan e KO CLINIC for Antiaging. 4-54 Onoecho, Naka-ku Yokohama-shi, Kanagawa 231-0015, Japan f Department of Plastic and Reconstructive Surgery, Yokohama City University Hospital. 3-9 Fukuura, Kanazawa-ku, Yokohama-shi, Kanagawa 236-0004, Japan Received 27 August 2018; accepted 6 January 2019 KEYWORDS Summary Eyebrow descent commonly occurs after ptosis repair or blepharoplasty surgery. Müller’s muscle; The procedures used to correct acquired blepharoptosis are primarily classified into four Eyebrow position; groups. These procedures target the levator aponeurosis, Müller’s muscle, both the aponeu- Blepharoptosis; rosis and Müller’s muscle, or the frontalis muscle. In this study, we used a new technique called MRD; external Müller’s muscle tucking (EMMT) on 51 patients (94 eyelids), which targets the Müller’s Ptosis repair muscle for involutional blepharoptosis. The patients were assessed by comparative analysis us- ing pre- and post-operative digital photographs. -

Conjunctival Flora of Normal Human Eye Which Vary with Age, Sex, Geographical Distribution, Right and Left Eye
Central JSM Ophthalmology Research Article *Corresponding author Purnima Rajkarnikar Sthapit, Department of Ophthalmology, Dhulikhel Hospital, Kathmandu Conjunctival Flora of Normal University Hospital, Dhulikhel, Kavre, Nepal, Tel: 009779813254962; Fax: 0097711490707; Email: Human Eye Submitted: 23 February 2014 Purnima Rajkarnikar Sthapit1* and Nhuchhe Ratna Tuladhar2 Accepted: 03 March 2014 1Department of Ophthalmology, Kathmandu University School of Medical Sciences, Nepal Published: 07 March 2014 2Department of Microbiology, Kathmandu University School of Medical Sciences, Nepal ISSN: 2333-6447 Copyright Abstract © 2014 Sthapit et al. Background: The normal flora of the eye plays an important role in maintaining OPEN ACCESS ocular homeostasis by various mechanisms. They comprise of mainly bacteria which do not cause infection in normal conditions but can be a main source of infection after Keywords ocular surgery, trauma or in immune compromised. The ranges of these microorganisms • Coagulase positive Staphylococcus vary with age, sex and geographical distribution. Therefore it is very important for the • Normal flora ophthalmologist to know the ocular normal flora before giving prophylactic antibiotics • Ocular infection and treating infections. • Ocular trauma Objectives: To describe the conjunctival flora of normal human eye which vary with age, sex, geographical distribution, right and left eye. Methodology: A total of 200 conjunctival swabs from 100 patients with healthy eyes were sent for microbiological evaluation to describe the various microorganisms isolated as normal flora of conjunctiva. Result: The growth of bacteria was seen in 78.5% of patients, the commonest flora isolated was Coagulase negative Staphylocccus in 51%. Greater number of male patients had sterile conjunctiva than females and conjunctiva of old people were found to be increasingly more colonised than young. -

Biorxives Gaze-Stabilization Pdf Creator
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.30.454479; this version posted August 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Visuo-vestibular gaze control – conserved subcortical processing Tobias Wibble1,2, Tony Pansell2, Sten Grillner1, Juan Pérez-Fernández1,3,* 1The Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden 2The Department of Clinical Neuroscience, Marianne Bernadotte Centrum, St: Erik’s Eye Hospital, Karolinska Institutet, Stockholm, Sweden 3CINBIO, Universidade de Vigo, Campus universitario Lagoas, Marcosende, 36310 Vigo, Spain *Corresponding author: [email protected] Abstract Gaze stabilization compensates for movements of the head or external environment to minimize image blurring, which is critical for visually-guided behaviors. Multisensory information is used to stabilize the visual scene on the retina via the vestibulo-ocular (VOR) and optokinetic (OKR) reflexes. While the organization of neuronal circuits underlying VOR is well described across vertebrates, less is known about the contribution and evolutionary origin of the OKR circuits. Moreover, the integration of these two sensory modalities is still poorly understood. Here, we developed a novel experimental model, the isolated lamprey eye-brain-labyrinth preparation, to analyze the neuronal pathways underlying visuo-vestibular integration which allowed electrophysiological recordings while applying vestibular stimulation using a moving platform, coordinated with visual stimulation via two screens. We show that lampreys exhibit robust visuo-vestibular integration, with optokinetic information processed in the pretectum 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.30.454479; this version posted August 1, 2021. -

Visual Pathways
Visual Pathways Michael Davidson Professor, Ophthalmology Diplomate, American College of Veterinary Ophthalmologists Department of Clinical Sciences College of Veterinary Medicine North Carolina State University Raleigh, North Carolina, USA <[email protected]> Vision in Animals Miller PE, Murphy CJ. Vision in Dogs. JAVMA. 1995; 207: 1623. Miller PE, Murphy CJ. Equine Vision. In Equine Ophthalmology ed. Gilger BC. 2nd ed. 2011: pp 398- 433. Ofri R. Optics and Physiology of Vision. In Veterinary Ophthalmology. ed. Gelatt KN 5th ed. 2013: 208-270, Visual Pathways, Responses and Reflexes: Relevant Structures Optic n (CN II) – somatic afferent Oculomotor n (CN III), Trochlear n (CN IV), Abducens n (CN VI) – somatic efferent to extraocular muscles Facial n (CN VII)– visceral efferent to eyelids Rostral colliculi – brainstem center that mediates somatic reflexes in response to visual stimuli Cerebellum Cerebro-cortex esp. occipital lobe www.studyblue.com Visual Pathway Visual Cortex Optic Radiation Lateral Geniculate Body www.studyblue.com Visual Field each cerebral hemisphere receives information from contralateral visual field (“the area that can be seen when the eye is directed forward”) visual field Visual Fiber (Retinotopic) Segregation nasal retinal fibers decussate at chiasm, temporal retinal fibers remain ipsilateral Nasal Temporal Retina Retina Fibers Fibers Decussate Remain Ipsilateral OD Visual Field OS Total visual field OD temporal nasal hemifield hemifield temporal nasal fibers fibers Nasal Temporal Retina = Retina = Temporal -
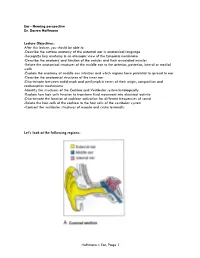
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Eyelid Conjunctival Tumors
EYELID &CONJUNCTIVAL TUMORS PHOTOGRAPHIC ATLAS Dr. Olivier Galatoire Dr. Christine Levy-Gabriel Dr. Mathieu Zmuda EYELID & CONJUNCTIVAL TUMORS 4 EYELID & CONJUNCTIVAL TUMORS Dear readers, All rights of translation, adaptation, or reproduction by any means are reserved in all countries. The reproduction or representation, in whole or in part and by any means, of any of the pages published in the present book without the prior written consent of the publisher, is prohibited and illegal and would constitute an infringement. Only reproductions strictly reserved for the private use of the copier and not intended for collective use, and short analyses and quotations justified by the illustrative or scientific nature of the work in which they are incorporated, are authorized (Law of March 11, 1957 art. 40 and 41 and Criminal Code art. 425). EYELID & CONJUNCTIVAL TUMORS EYELID & CONJUNCTIVAL TUMORS 5 6 EYELID & CONJUNCTIVAL TUMORS Foreword Dr. Serge Morax I am honored to introduce this Photographic Atlas of palpebral and conjunctival tumors,which is the culmination of the close collaboration between Drs. Olivier Galatoire and Mathieu Zmuda of the A. de Rothschild Ophthalmological Foundation and Dr. Christine Levy-Gabriel of the Curie Institute. The subject is now of unquestionable importance and evidently of great interest to Ophthalmologists, whether they are orbital- palpebral specialists or not. Indeed, errors or delays in the diagnosis of tumor pathologies are relatively common and the consequences can be serious in the case of malignant tumors, especially carcinomas. Swift diagnosis and anatomopathological confirmation will lead to a treatment, discussed in multidisciplinary team meetings, ranging from surgery to radiotherapy. -

Lmmunohistochemical Localization of Neuronal Nicotinic Receptors in the Rodent Central Nervous System
The Journal of Neuroscience, October 1987, 7(10): 3334-3342 lmmunohistochemical Localization of Neuronal Nicotinic Receptors in the Rodent Central Nervous System L. W. Swanson,1v2 D. M. Simmons, I12 P. J. Whiting,’ and J. Lindstrom’ ‘The Salk Institute for Biological Studies, and 2Howard Hughes Medical Institute, La Jolla, California 92037 The distribution of nicotinic acetylcholine receptors (AChR) are structurally unrelated (Kubo et al., 1986a, b) to nicotinic in the rat and mouse central nervous system has been ACh receptors (AChR, Noda et al., 1983a, b), which act by mapped in detail using monoclonal antibodies to receptors regulating directly the opening of a cation channel that is an purified from chicken and rat brain. Initial studies in the intrinsic component of the molecule. Furthermore, subtypes of chicken brain indicate that different neuronal AChRs are con- neuronal AChRs have been identified on the basis of pharma- tained in axonal projections to the optic lobe in the midbrain cological and structural properties (Whiting et al., 1987a). To from neurons in the lateral spiriform nucleus and from retinal understand the functional significanceof ACh in a particular ganglion cells. Monoclonal antibodies to the chicken and rat neural system, it is therefore necessaryto establishthe cellular brain AChRs also label apparently identical regions in all localization of ACh, and the cellular localization and type of major subdivisions of the central nervous system of rats and cholinergic receptor with which it interacts. Immunohistochem- mice, and this pattern is very similar to previous reports of istry provides a sensitive method for localizing cholinergic neu- 3H-nicotine binding, but quite different from that of a-bun- rons with antibodies to the synthetic enzyme choline acetyl- garotoxin binding. -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

A Rare Presentation of Lower Conjunctival Fornix Eyelashes Cyst Dr Mohammad Aldroos (MD)* *Department of Ophthalmology, Jordanian Royal Medical Services
Int J Biol Med Res.2018 ;9(1):6259-6260 Int J Biol Med Res www.biomedscidirect.com Volume 6, Issue 2, April 2015 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research Journal homepage: www.biomedscidirect.com BioMedSciDirect International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Case report A rare presentation of lower conjunctival fornix eyelashes cyst Dr Mohammad Aldroos (MD)* *Department of Ophthalmology, Jordanian Royal Medical Services A R T I C L E I N F O A B S T R A C T Keywords: Purpose: To describe a rare presentation of a right lower conjunctival fornix eyelashes cyst. Case Report: A 56 year-old lady presented to our clinic complaining of blackish mass in the lower conjunctival fornix of the right eye ( see fig.1), with mild conjunctival hyperemia around the mass. There was no history of discharge and difficulty in extraocular muscles movements also there was no history of trauma. A gradually enlarging mass over the past few years with recurrent conjunctivitis. Slit lamp examination of the mass showed normal appearing eyelashes surrounded by capsule only. The mass was surgically excised with good cosmetic appearance and without any complications c Copyright 2010 BioMedSciDirect Publications IJBMR - ISSN: 0976:6685. All rights reserved. Introduction Fig.2 Eyelashes grow on the edge of the eyelid. A normal eyelid has a single row of eyelashes located along its anterior margin. The posterior portion of the lid contains a row of Meibomian glands orifices, which secrete the oily component of the tear film (1). There are approximately 100 eye lashes on the upper lid and approximately 50-75 on the lower lid, they protect the eyeball. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.