Indications for Lumbar Total Disc Replacement: Selecting the Right Patient with the Right Indication for the Right Total Disc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
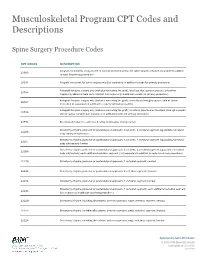
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020)
Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020) Procedure Code Description ACL Repair 27407 Repair, primary, torn ligament and/or capsule, knee; cruciate ACL Repair 27409 Repair, primary, torn ligament and/or capsule, knee; collateral and cruciate ligaments ACL Repair 29888 Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction Acromioplasty and Rotator Cuff Repair 23130 Acromioplasty Or Acromionectomy, Partial, With Or Without Coracoacromial Ligament Release Acromioplasty and Rotator Cuff Repair 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Acromioplasty and Rotator Cuff Repair 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Acromioplasty and Rotator Cuff Repair 23415 Coracoacromial Ligament Release, With Or Without Acromioplasty Acromioplasty and Rotator Cuff Repair 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Arthroscopy, Shoulder, Surgical; Decompression Of Subacromial Space With Partial Acromioplasty, With Coracoacromial Ligament (Ie, Arch) Release, When Performed (List Separately In Addition Acromioplasty and Rotator Cuff Repair 29826 To Code For Primary Procedure) Acromioplasty and Rotator Cuff Repair 29827 Arthroscopy, shoulder, surgical; with rotator cuff repair Allograft for Spinal Fusion [BMP] 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only Ankle Fusion 27870 Arthrodesis, ankle, open Ankle Fusion -

Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy
Y Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines 1 G Evidence-Based Clinical Guidelines for Multidisciplinary ETHODOLO Spine Care M NE I DEL I U /G ON Diagnosis and Treatment of I NTRODUCT Lumbar Disc I Herniation with Radiculopathy NASS Evidence-Based Clinical Guidelines Committee D. Scott Kreiner, MD Paul Dougherty, II, DC Committee Chair, Natural History Chair Robert Fernand, MD Gary Ghiselli, MD Steven Hwang, MD Amgad S. Hanna, MD Diagnosis/Imaging Chair Tim Lamer, MD Anthony J. Lisi, DC John Easa, MD Daniel J. Mazanec, MD Medical/Interventional Treatment Chair Richard J. Meagher, MD Robert C. Nucci, MD Daniel K .Resnick, MD Rakesh D. Patel, MD Surgical Treatment Chair Jonathan N. Sembrano, MD Anil K. Sharma, MD Jamie Baisden, MD Jeffrey T. Summers, MD Shay Bess, MD Christopher K. Taleghani, MD Charles H. Cho, MD, MBA William L. Tontz, Jr., MD Michael J. DePalma, MD John F. Toton, MD This clinical guideline should not be construed as including all proper methods of care or excluding or other acceptable methods of care reason- ably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment is to be made by the physi- cian and patient in light of all circumstances presented by the patient and the needs and resources particular to the locality or institution. I NTRODUCT 2 Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines I ON Financial Statement This clinical guideline was developed and funded in its entirety by the North American Spine Society (NASS). All participating /G authors have disclosed potential conflicts of interest consistent with NASS’ disclosure policy. -

Commercial Musculoskeletal Codes
Updated January 2018 Commercial Musculoskeletal Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery Codes associated with an Arthrogram CPT Description Commercial Notes Partial excision of posterior vertebral component (eg, spinous 22100 process, lamina or facet) for intrinsic bony lesion, single vertebral segment; cervical 22101 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; thoracic 22102 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; lumbar Partial excision of posterior vertebral component (eg, spinous process, 22103 lamina or facet) for intrinsic bony lesion, single vertebral segment; each additional segment (List separately in addition to code for primary procedure) Partial excision of vertebral body, for intrinsic bony lesion, without 22110 decompression of spinal cord or nerve root(s), single vertebral segment;cervical Partial excision of vertebral body, for intrinsic bony lesion, without 22112 decompression of spinal cord or nerve root(s), single vertebral segment; thoracic Partial excision of vertebral body, for intrinsic bony lesion, without 22114 decompression of spinal cord or nerve root(s), single vertebral segment; lumbar each additional vertebral segment (list separately in addition to code 22116 for primary procedure) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 22206 1 vertebral -
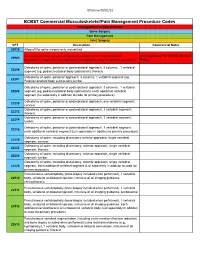
Commercial MSK Procedure Code List Effective 09.01.21.Xlsx
Effective 09/01/21 BCBST Commercial Musculoskeletal/Pain Management Procedure Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery CPT Description Commercial Notes 20930 Allograft for spine surgery only morselized Computer-assisted surgical navigational procedure for musculoskeletal Investigational Per BCBST Medical 20985 procedures, image-less (List separately in addition to code for primary procedure) Policy Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22206 segment (eg, pedicle/vertebral body subtraction); thoracic Osteotomy of spine, posterior approach, 3 columns, 1 vertebral segment (eg. 22207 Pedicle/vertebral body subtraction);lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22208 segment (eg, pedicle/vertebral body subtraction); each additional vertebral segment (list separately in addition to code for primary procedure) Osteotomy of spine, posterior or posterolateral approach, one vertebral segment; 22210 cervical Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22212 thoracic Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22214 lumbar Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22216 each additional vertebral segment (List separately in addition to primary procedure) Osteotomy of spine, including discectomy anterior approach, single vertebral 22220 segment; cervical Osteotomy of spine, including discectomy, anterior approach, single -

The Comprehensive Anatomical Spinal Osteotomy and Anterior Column Realignment Classification
CLINICAL ARTICLE J Neurosurg Spine 29:565–575, 2018 The comprehensive anatomical spinal osteotomy and anterior column realignment classification Presented at the 2018 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Juan S. Uribe, MD,1 Frank Schwab, MD,2 Gregory M. Mundis Jr., MD,3 David S. Xu, MD,1 Jacob Januszewski, DO,4 Adam S. Kanter, MD,5 David O. Okonkwo, MD, PhD,5 Serena S. Hu, MD,6 Deviren Vedat, MD,7 Robert Eastlack, MD,3 Pedro Berjano, MD, PhD,8 and Praveen V. Mummaneni, MD9 1Department of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona; 2Department of Orthopedic Surgery, Hospital for Special Surgery, New York, New York; 3San Diego Spine Foundation, La Jolla, California; 4Orlando Neurosurgery, Orlando, Florida; 5Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania; 6Department of Orthopaedic Surgery, Stanford School of Medicine, Stanford, California; Departments of 7Orthopaedic Surgery and 9Neurological Surgery, University of California, San Francisco, California; and 8IRCCS Istituto Ortopedico Galeazzi, Milan, Italy OBJECTIVE Spinal osteotomies and anterior column realignment (ACR) are procedures that allow preservation or restoration of spine lordosis. Variations of these techniques enable different degrees of segmental, regional, and global sagittal realignment. The authors propose a comprehensive anatomical classification system for ACR and its variants based on the level of technical complexity and invasiveness. This serves as a common language and platform to stan- dardize clinical and radiographic outcomes for the utilization of ACR. METHODS The proposed classification is based on 6 anatomical grades of ACR, including anterior longitudinal liga- ment (ALL) release, with varying degrees of posterior column release or osteotomies. -

2018 Nuvasive® Reimbursement Guide
2018 NuVasive® Reimbursement Guide Assisting physicians and facilities in accurate billing for NuVasive implants and instrumentation systems. 2018 Reimbursement Guide Contents I. Introduction .......................................................................................................................................................................2 II. Physician Coding and Payment ......................................................................................................................................2 Fusion Facilitating Technologies ............................................................................................................................................. 2 NVM5® Intraoperative Monitoring System ........................................................................................................................... 12 III. Hospital Inpatient Coding and Payment ....................................................................................................................13 NuVasive® Technology ..........................................................................................................................................................13 Non-Medicare Reimbursement ............................................................................................................................................13 IV. Outpatient Facility Coding and Payment ..................................................................................................................14 Hospital Outpatient -

Description of Procedure Price ARTHROSCOPY, SHOULDER
ORTHOPEDIC PROCEDURES- PROFESSIONAL FEES ONLY Description of Procedure Price ARTHROSCOPY, SHOULDER, GLENOHUMERAL JT $ 4,152 CLAVICULAR FX, ORIF $ 2,054 BIOPSY, SOFT TISSUE, UPPER ARM/ELBOW $ 1,155 EXCISION, TUMOR, SOFT TISSUE OF UPPER ARM OR ELBOW $ 1,099 DEBRIDEMENT MUSCLE FASCIA < 20 SQ CM $ 444 REINSERTION, RUPTURED BICEPS/TRICPS TEND $ 2,207 REPAIR LATERAL COLLATERAL LIGAMENT, ELBOW $ 1,917 DEBRIDE LAT/MED ELBOW $ 1,494 TENOTOMY, ELBOW, LATERAL OR MEDIAL (EG, EPICONDYLITIS, TENNIS ELBOW, GOLFER'S ELBOW); DEBRIDEMENT, SOFT TISSUE AND/OR BONE, OPEN WITH TENDON REPAIR OR REATTACHMENT $ 1,785 DEBRIDEMENT BONE MUSCLE/FASCIA <20 SQCM $ 659 INCISION, EXTENSOR TENDON SHEATH, WRIS $ 912 DECOMPRESSION FASCIOTOMY, FOREARM AND/OR WRIST $ 1,545 DEBRIDEMENT BONE EA ADDL 20 SQCM $ 285 EXCISION OR CURETTAGE OF BONE CYST OR BENIGN TUMOR $ 1,502 CARPECTOMY; ALL BONES OF PROXIMAL ROW $ 1,668 REPAIR OF NONUNION OR MALUNION, RADIUS OR ULNA $ 2,170 ARTHROPLASTY, INTERPOSITION, INTERCARPAL OR CPM $ 2,237 INCISIONAL BIOPSY OF SKIN (EG, WEDGE) $ 163 OPEN TRMT OF DISTAL RADIAL FX $ 2,102 ORIF DISTAL RADIOUS INTRA-ARTICULAR $ 2,347 TENDON SHEATH INCISION; FINGERS/HAND $ 874 REPAIR EXTENSOR TENDON WO GRAFT $ 1,597 TRANSFER OR TRANSPLANT OF TENDON, CARPOMETACARPAL $ 1,988 PERCUTANEOUS SKELETAL FIX UNSTABLE PHALANGE $ 1,333 OPEN TX PHALANGEAL FX $ 1,710 AMPUTATION, FINGER OR THUMB, PRIMARY OR SECONDARY $ 1,710 HIP ARTHROPLASTY $ 3,884 REVISION, TOTAL HIP $ 5,490 REVISION, THA, ACETABULUM $ 4,219 REVISON, THA, FEMUR $ 4,383 UNSPECIFIED PROCEDURE ON HIP $ 2,673 -

Laminectomy, Facetectomy, Discectomy, Laminotomy, Foraminotomy
www.caryortho.com www.mathurspinesurgery.com Instructions for before and after Surgery Laminectomy, Facetectomy, Discectomy, Laminotomy, Foraminotomy Before You Have Surgery Do not eat or drink after midnight the night before surgery. Please take all routine medications the day of surgery unless otherwise directed by Dr. Mathur, his Nurse (Melissa), or Hospital Staff. Medications to stop prior to surgery: ________________________________ _______________________________________ ________________________________ _______________________________________ You may stay overnight in the hospital. Please bring your brace to the hospital the day of surgery. While you recover at home it is important you protect your spine as it heals. This can best be achieved by following the below instructions and contacting your nurse with any questions or concerns. When You Return Home Your initial activity level will be influenced by the anesthetic agent you have received. It is not uncommon to feel drowsy or tired for a number of hours. Rest when you need it. Symptoms may peak at 2-5 days after surgery, and then will begin to subside. Diet: • Return to your normal diet slowly as tolerated. • Calories and protein are very important for the healing process. Restricting these is not recommended during this time. Back Brace: • Remove your brace to sleep and shower. • You will wear your brace when sitting or standing longer than 10minutes. • You are to wear your back brace until you come to your first post op appointment. At this time we will discuss further use of the brace. It is commonly needed until 6 weeks after surgery. • The purpose of your brace is to stabilize your back to allow for superior healing.