Minimally Invasive and Laser Spine Procedures These Services May Or May Not Be Covered by Your Healthpartners Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anterior Reconstruction Techniques for Cervical Spine Deformity
Neurospine 2020;17(3):534-542. Neurospine https://doi.org/10.14245/ns.2040380.190 pISSN 2586-6583 eISSN 2586-6591 Review Article Anterior Reconstruction Techniques Corresponding Author for Cervical Spine Deformity Samuel K. Cho 1,2 1 1 1 https://orcid.org/0000-0001-7511-2486 Murray Echt , Christopher Mikhail , Steven J. Girdler , Samuel K. Cho 1Department of Orthopedics, Icahn School of Medicine at Mount Sinai, New York, NY, USA Department of Orthopaedics, Icahn 2 Department of Neurological Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, School of Medicine at Mount Sinai, 425 NY, USA West 59th Street, 5th Floor, New York, NY, USA E-mail: [email protected] Cervical spine deformity is an uncommon yet severely debilitating condition marked by its heterogeneity. Anterior reconstruction techniques represent a familiar approach with a range Received: June 24, 2020 of invasiveness and correction potential—including global or focal realignment in the sagit- Revised: August 5, 2020 tal and coronal planes. Meticulous preoperative planning is required to improve or prevent Accepted: August 17, 2020 neurologic deterioration and obtain satisfactory global spinal harmony. The ability to per- form anterior only reconstruction requires mobility of the opposite column to achieve cor- rection, unless a combined approach is planned. Anterior cervical discectomy and fusion has limited focal correction, but when applied over multiple levels there is a cumulative ef- fect with a correction of approximately 6° per level. Partial or complete corpectomy has the ability to correct sagittal deformity as well as decompress the spinal canal when there is an- terior compression behind the vertebral body. -

Spinal Interventional Pain Management and Lumbar Spine Surgery
Spinal Interventional Pain Management and Lumbar Spine Surgery Policy Number: Original Effective Date: MM.06.024 01/01/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/15/2017 Section: Surgery; Medicine Place(s) of Service: Office; Outpatient; Inpatient I. Description The following spinal interventional pain management and lumbar spine surgery procedures require precertification through Magellan Hawaii, formally known as National Imaging Associates, Inc. (NIA): A. Spinal Epidural Injections B. Paravertebral Facet Joint Denervation (radiofrequency neurolysis) C. Paravertebral Facet Joint Injections or Blocks D. Sacroiliac joint injections E. Lumbar Spinal Fusion Surgery II. Administrative Guidelines A. The ordering physician can obtain precertification or consult with Magellan Hawaii by accessing their website at http://www.radmd.com/ or by calling 1 (866) 306-9729, from 6 a.m. to 6 p.m., weekdays, Hawaii Time. Refer to the e-library for instructions on navigating the radmd.com website (RadMD Get Started) and requesting precertification/checking the status of a request (RadMD QuickStart). B. For access to the latest clinical guidelines used for precertification, go to www.radmd.com and click on the link entitled View Clinical Guidelines. C. For interventional pain management procedures (epidural injections, facet joint denervation neurolysis, facet joint injections and sacroiliac joint injections), if more than one procedure is planned, a separate precertification number must be obtained for each procedure planned. D. For spinal surgeries (lumbar fusions, lumbar decompressions, and lumbar microdiscectomy), one precertification number should be obtained for the most invasive surgery to be performed. E. Precertification requirements for injection procedures apply only to office and outpatient services (POS 11, 22, or 24). -

Lumbar Spine Schmorl's Nodes; Prevalence in Adults with Back Pain, and Their Relation to Vertebral Endplate Degeneration Israa Mohammed Sadiq
Sadiq Egyptian Journal of Radiology and Nuclear Medicine (2019) 50:65 Egyptian Journal of Radiology https://doi.org/10.1186/s43055-019-0069-9 and Nuclear Medicine RESEARCH Open Access Lumbar spine Schmorl's nodes; prevalence in adults with back pain, and their relation to vertebral endplate degeneration Israa Mohammed Sadiq Abstract Background: In 1927, Schmorl described a focal herniation of disc material into the adjacent vertebral body through a defect in the endplate, named as Schmorl’s node (SN). The aim of the study is to reveal the prevalence and distribution of Schmorl’s nodes (SNs) in the lumbar spine and their relation to disc degeneration disease in Kirkuk city population. Results: A cross-sectional analytic study was done for 324 adults (206 females and 118 males) with lower back pain evaluated as physician requests by lumbosacral MRI at the Azadi Teaching Hospital, Kirkuk city, Iraq. The demographic criteria of the study sample were 20–71 years old, 56–120 kg weight, and 150–181 cm height. SNs were seen in 72 patients (22%). Males were affected significantly more than the females (28.8% vs. 18.8%, P = 0.03). SNs were most significantly affecting older age groups. L1–L2 was the most affected disc level (23.6%) and the least was L5–S1 (8.3%). There was neither a significant relationship between SN and different disc degeneration scores (P = 0.76) nor with disc herniation (P = 0.62, OR = 1.4), but there was a significant relation (P = 0.00001, OR = 7.9) with MC. Conclusion: SN is a frequent finding in adults’ lumbar spine MRI, especially in males; it is related to vertebral endplate bony pathology rather than discal pathology. -

Automated Percutaneous and Endoscopic Discectomy
Corporate Medical Policy Automated Percutaneous and Endoscopic Discectomy File Name: percutaneous_discectomy Origination: 9/1991 Last CAP Review: 5/2021 Next CAP Review: 5/2022 Last Review: 5/2021 Description of Procedure or Service Surgical management of herniated intervertebral discs most commonly involves discectomy or microdiscectomy, performed manually through an open incision. Automated percutaneous discectomy involves placement of a probe within the intervertebral disc under image guidance with aspiration of disc material using a suction cutting device. Removal of disc herniations under endoscopic visualization is also being investigated. Endoscopic discectomy involves the percutaneous placement of a working channel under image guidance, followed by visualization of the working space and instruments through an endoscope, and aspiration of disc material. Back pain or radiculopathy related to herniated discs is an extremely common condition and a frequent cause of chronic disability. Although many cases of acute low back pain and radiculopathy will resolve with conservative care, a surgical decompression is often considered when the pain is unimproved after several months and is clearly neuropathic in origin, resulting from irritation of the nerve roots. Open surgical treatment typically consists of discectomy, in which the extruding disc material is excised. When performed with an operating microscope the procedure is known as microdiscectomy. Minimally invasive options have also been researched, in which some portion of the disc is removed or ablated, although these techniques are not precisely targeted at the offending extruding disc material. Ablative techniques include laser discectomy and radiofrequency (RF) decompression. In addition, intradiscal electrothermal annuloplasty is another minimally invasive approach to low back pain. -

I Think with Flying Colours, Express
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.50.9.1251 on 1 September 1987. Downloaded from Book reviews 1251 The eight biographies of disabled people differences. In the chapter on neirve blocking Intensive Care and Monitoring of the Neuro- with which the book begins demonstrate the procedures it is most refreshing tto see Charl- surgical. Patient (Progress in Neurological author's skill in empathising with individu- ton's cautious approach, criticalI analysis of Surgery Vol 12.) Edited by AM Landolt. als and outlining important aspects of aging methods and results, and warni ngs ("before (Pp 202; $74.00.) Basel: Karger, 1987. with disability. It is sad that there is no bet- embarking upon neurolytic block everyone ter book covering this topic. Why not? As should read the review of rnedico-legal It is unfortunate that this volume, dedicated the author says, saving lives attracts glam- aspects of complications that may follow to Hugo Krayenbuhl with such a good our and funds which are denied to services these procedures"). He states that intra- biography, has suffered the vicissitudes of for the saved. If this is true of spinal injury thecal use ofcold hypertonic saliine and bar- multiple authorship (so frankly acknowl- and poliomyelitis it is no less true of the botage of CSF "can now be regaLrded as his- edged in the editors' preface that it could be beneficiaries ofother miracles: for example a torical curiosities"; of epidural lblock "there taken as a lament upon this type of book); generation of people with spina bifida and is a distinct lack of published (data on the relatively little of the title is covered by the hydrocephalus now being launched into an injection of neurolytic solutions into the epi- contents, and the relationship of the eight adult world which has made no special dural space"; and neurolytic p;aravertebral contributions is seemingly haphazard. -
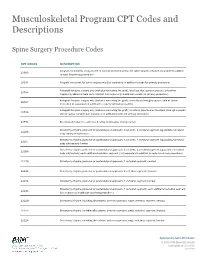
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Neurosurgery
March 10, 2017 St Elizabeth Healthcare 9:02 am Privileges for: Neurosurgery Request ST. ELIZABETH - EDGEWOOD ST. ELIZABETH - FLORENCE ST. ELIZABETH - FT. THOMAS ST. ELIZABETH - GRANT CO. (Surgical & other invasive procedures requiring general anesthetic are not offered) MEC Approval: August 27, 2009; Rev. November 15, 2012, February 27, 2014 Board Approval: September 14, 2009; Rev. January 7, 2013, May 5, 2014 DEPARTMENT APPROVAL ________Approved ________Disapproved ___________________________________________ ________________ Department/Section Chair Signature Date MINIMUM REQUIREMENTS Degree required: MD or DO Successful completion of an ACGME or AOA accredited residency in neurosurgery. Note: For Practitoners (excluding AHPs) who apply for membership after March 2, 2009 be and remain (with a lapse of no longer than one year) board certified in their principal practice specialty, or become and remain (with a lapse of no longer than one year) board certified within six years of completion of their post-graduate medical training. Only those boards recognized by the American Board of Medical Specialties or the American Osteopathic Association are acceptable. This board certification requirement does not apply to applicants who on March 2, 2009 were members in good standing on the medical staff of the St. Luke Hospitals or St. Elizabeth Medical Center. PRIVILEGES REQUESTED Pursuant to Bylaws Section 6.1.4, practitioners may exercise the privileges requested and awarded below only at facilities where St. Elizabeth Healthcare offers those services. I. Core Privileges: Core privileges in neurosurgery include the care, treatment or services listed immediately below. I specifically acknowledge that board certification alone does not necessarily qualify me to perform all core privileges or assure competence in all clinical areas. -
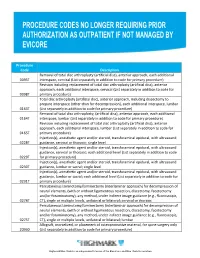
Procedure Codes No Longer Requiring Prior
Procedure Code Description Removal of total disc arthroplasty (artificial disc), anterior approach, each additional 0095T interspace, cervical (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, cervical (List separately in addition to code for 0098T primary procedure) Total disc arthroplasty (artificial disc), anterior approach, including discectomy to prepare interspace (other than for decompression), each additional interspace, lumbar 0163T (List separately in addition to code for primary procedure) Removal of total disc arthroplasty, (artificial disc), anterior approach, each additional 0164T interspace, lumbar (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, lumbar (List separately in addition to code for 0165T primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0228T guidance, cervical or thoracic; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound guidance, cervical or thoracic; each additional level (List separately in addition to code 0229T for primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0230T guidance, lumbar or sacral; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Artificial Intervertebral Disc: Lumbar Spine Page 1 of 22
Artificial Intervertebral Disc: Lumbar Spine Page 1 of 22 Medical Policy An Independent licensee of the Blue Cross Blue Shield Association Title: Artificial Intervertebral Disc: Lumbar Spine Related Policy: . Artificial Intervertebral Disc: Cervical Spine Professional Institutional Original Effective Date: August 9, 2005 Original Effective Date: August 9, 2005 Revision Date(s): June 14, 2006; Revision Date(s): June 14, 2006; January 1, 2007; July 24, 2007; January 1, 2007; July 24, 2007; September 25 2007; February 22, 2010; September 25 2007; February 22, 2010; March 10, 2011; March 8, 2013; March 10 2011; March 8, 2013; June 23, 2015; August 4, 2016; June 23, 2015; August 4, 2016; May 23, 2018; July 17, 2019; May 23, 2018; July 17, 2019; August 21, 2020; July 1, 2021 August 21, 2020; July 1, 2021 Current Effective Date: September 23, 2008 Current Effective Date: September 23, 2008 State and Federal mandates and health plan member contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. To verify a member's benefits, contact Blue Cross and Blue Shield of Kansas Customer Service. The BCBSKS Medical Policies contained herein are for informational purposes and apply only to members who have health insurance through BCBSKS or who are covered by a self-insured group plan administered by BCBSKS. Medical Policy for FEP members is subject to FEP medical policy which may differ from BCBSKS Medical Policy. The medical policies do not constitute medical advice or medical care. Treating health care providers are independent contractors and are neither employees nor agents of Blue Cross and Blue Shield of Kansas and are solely responsible for diagnosis, treatment and medical advice. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID