Laminectomy, Facetectomy, Discectomy, Laminotomy, Foraminotomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spinal Interventional Pain Management and Lumbar Spine Surgery
Spinal Interventional Pain Management and Lumbar Spine Surgery Policy Number: Original Effective Date: MM.06.024 01/01/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/15/2017 Section: Surgery; Medicine Place(s) of Service: Office; Outpatient; Inpatient I. Description The following spinal interventional pain management and lumbar spine surgery procedures require precertification through Magellan Hawaii, formally known as National Imaging Associates, Inc. (NIA): A. Spinal Epidural Injections B. Paravertebral Facet Joint Denervation (radiofrequency neurolysis) C. Paravertebral Facet Joint Injections or Blocks D. Sacroiliac joint injections E. Lumbar Spinal Fusion Surgery II. Administrative Guidelines A. The ordering physician can obtain precertification or consult with Magellan Hawaii by accessing their website at http://www.radmd.com/ or by calling 1 (866) 306-9729, from 6 a.m. to 6 p.m., weekdays, Hawaii Time. Refer to the e-library for instructions on navigating the radmd.com website (RadMD Get Started) and requesting precertification/checking the status of a request (RadMD QuickStart). B. For access to the latest clinical guidelines used for precertification, go to www.radmd.com and click on the link entitled View Clinical Guidelines. C. For interventional pain management procedures (epidural injections, facet joint denervation neurolysis, facet joint injections and sacroiliac joint injections), if more than one procedure is planned, a separate precertification number must be obtained for each procedure planned. D. For spinal surgeries (lumbar fusions, lumbar decompressions, and lumbar microdiscectomy), one precertification number should be obtained for the most invasive surgery to be performed. E. Precertification requirements for injection procedures apply only to office and outpatient services (POS 11, 22, or 24). -

Lumbar Laminectomy Or Laminotomy
Patient Instructions: Lumbar Laminectomy or Laminotomy Surgical Technique A lumbar laminectomy or laminotomy is a surgical approach performed from the back of the lumbar spine. It is usually done through an incision in the middle of the back. Using minimally invasive techniques a small window of bone is drilled in the lamina to allow the surgeon to unpinch the underlying nerves (laminotomy), or in more severe cases the bone is removed completely on both sides to allow nerves on both sides of the spinal canal to be decompressed (laminectomy). It is done using an operating microscope and microsurgical technique. It is used to treat spinal stenosis or lateral recess stenosis and alleviate the pain and/or numbness that occurs in a patients lower back or legs. It can many times be performed on an outpatient basis without the need for an overnight stay in a hospital. Before Surgery • Seven days prior to surgery, please do not take any anti-inflammatory NSAID medications (Celebrex, Ibuprofen, Aleve, Naprosyn, Advil, etc.) as this could prolong your bleeding time during surgery. • Do not eat or drink anything after midnight the day before surgery. This means nothing to drink the morning of surgery except you may take your normal medication with a sip of water if needed. This includes your blood pressure medicine, which in general should be taken. Consult your surgeon or primary care doctor regarding insulin if you take it. • Please do not be late to check in on the day of surgery or it may be cancelled. • Please bring your preoperative folder with you to the surgery and have it when you check in. -
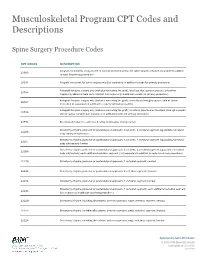
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -
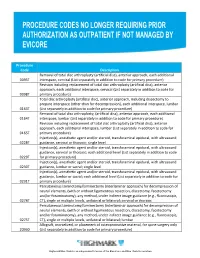
Procedure Codes No Longer Requiring Prior
Procedure Code Description Removal of total disc arthroplasty (artificial disc), anterior approach, each additional 0095T interspace, cervical (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, cervical (List separately in addition to code for 0098T primary procedure) Total disc arthroplasty (artificial disc), anterior approach, including discectomy to prepare interspace (other than for decompression), each additional interspace, lumbar 0163T (List separately in addition to code for primary procedure) Removal of total disc arthroplasty, (artificial disc), anterior approach, each additional 0164T interspace, lumbar (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, lumbar (List separately in addition to code for 0165T primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0228T guidance, cervical or thoracic; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound guidance, cervical or thoracic; each additional level (List separately in addition to code 0229T for primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0230T guidance, lumbar or sacral; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020)
Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020) Procedure Code Description ACL Repair 27407 Repair, primary, torn ligament and/or capsule, knee; cruciate ACL Repair 27409 Repair, primary, torn ligament and/or capsule, knee; collateral and cruciate ligaments ACL Repair 29888 Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction Acromioplasty and Rotator Cuff Repair 23130 Acromioplasty Or Acromionectomy, Partial, With Or Without Coracoacromial Ligament Release Acromioplasty and Rotator Cuff Repair 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Acromioplasty and Rotator Cuff Repair 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Acromioplasty and Rotator Cuff Repair 23415 Coracoacromial Ligament Release, With Or Without Acromioplasty Acromioplasty and Rotator Cuff Repair 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Arthroscopy, Shoulder, Surgical; Decompression Of Subacromial Space With Partial Acromioplasty, With Coracoacromial Ligament (Ie, Arch) Release, When Performed (List Separately In Addition Acromioplasty and Rotator Cuff Repair 29826 To Code For Primary Procedure) Acromioplasty and Rotator Cuff Repair 29827 Arthroscopy, shoulder, surgical; with rotator cuff repair Allograft for Spinal Fusion [BMP] 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only Ankle Fusion 27870 Arthrodesis, ankle, open Ankle Fusion -

Posterior Foraminotomy Versus Anterior Decompression and Fusion
CLINICAL ARTICLE J Neurosurg Spine 32:344–352, 2020 Posterior foraminotomy versus anterior decompression and fusion in patients with cervical degenerative disc disease with radiculopathy: up to 5 years of outcome from the national Swedish Spine Register Anna MacDowall, MD, PhD,1 Robert F. Heary, MD,2 Marek Holy, MD,3 Lars Lindhagen, PhD,4 and Claes Olerud, MD, PhD1 1Department of Surgical Sciences, Uppsala University Hospital, Uppsala, Sweden; 2Department of Neurological Surgery, Rutgers New Jersey Medical School, Newark, New Jersey; 3Department of Orthopedics, Örebro University Hospital, Örebro; and 4Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden OBJECTIVE The long-term efficacy of posterior foraminotomy compared with anterior cervical decompression and fusion (ACDF) for the treatment of degenerative disc disease with radiculopathy has not been previously investigated in a population-based cohort. METHODS All patients in the national Swedish Spine Register (Swespine) from January 1, 2006, until November 15, 2017, with cervical degenerative disc disease and radiculopathy were assessed. Using propensity score matching, pa- tients treated with posterior foraminotomy were compared with those undergoing ACDF. The primary outcome measure was the Neck Disability Index (NDI), a patient-reported outcome score ranging from 0% to 100%, with higher scores indicating greater disability. A minimal clinically important difference was defined as > 15%. Secondary outcomes were assessed with additional patient-reported outcome measures (PROMs). RESULTS A total of 4368 patients (2136/2232 women/men) met the inclusion criteria. Posterior foraminotomy was performed in 647 patients, and 3721 patients underwent ACDF. After meticulous propensity score matching, 570 patients with a mean age of 54 years remained in each group. -

Posterior Cervical Day Surgery: Foraminotomy/Microdiscectomy Or Decompression
Posterior Cervical Day Surgery: Foraminotomy/Microdiscectomy or Decompression Information for patients and families Read this booklet to learn: • how to prepare • what you can do when you get home • how to care for your incision • what activities you can do • what problems to look out for • information about your follow-up appointments You will be going home the same day as your lumbar spine surgery. For 24 hours after your surgery: • Do not travel alone. Someone must pick you up from the hospital and take you home. • Do not drink alcohol. Krembil Neuroscience Centre Please visit the UHN Patient Education website for more health information: www.uhnpatienteducation.ca © 2016 University Health Network. All rights reserved. This information is to be used for informational purposes only and is not intended as a substitute for professional medical advice, diagnosis or treatment. Please consult your health care provider for advice about a specific medi- cal condition. A single copy of these materials may be reprinted for non-commercial personal use only. Author: Spinal Program Revised: 05/2016 Form: D-5213 Preparing for surgery How can I prepare for my surgery? □ Let your family and friends know that you may need some help when you get home. You will probably need help for a few weeks with things like cooking, grocery shopping, and housework. If you live alone, see if you can stay with family or friends for a while after surgery. □ Some medicines may increase your risk of bleeding during or after your procedure. Tell your doctor or health -

Vertebral Augmentation Involving Vertebroplasty Or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: a Systematic Review
Health Quality Ontario The provincial advisor on the quality of health care in Ontario ONTARIO HEALTH TECHNOLOGY ASSESSMENT SERIES Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review KEY MESSAGES Cancer can start in one part of the body and spread to other regions, often involving the spine, causing significant pain and reducing a patient’s ability to walk or carry out everyday activities such as bathing, dressing, and eating. When cancer spreads to or occurs in a bone of the spine (a vertebral bone), the cancer can weaken and break this bone. These fractures, if left untreated, can negatively affect the quality of life of terminally ill patients and their families. Vertebroplasty and kyphoplasty are two types of procedures called vertebral augmentation. During vertebral augmentation, the physician injects bone cement into the broken vertebral bone to stabilize the spine and control pain. Kyphoplasty is a modified form of vertebroplasty in which a small balloon is first inserted into the vertebral bone to create a space to inject the cement; it also attempts to lift the fracture to restore it to a more normal position. Medical therapy and bed rest are not very effective in cancer patients with painful vertebral fractures, and surgery is not usually an option for patients with advanced disease and who are in poor health. Vertebral augmentation is a minimally invasive treatment option, performed on an outpatient basis without general anesthesia, for managing painful vertebral fractures that limit mobility and self-care. We reviewed the evidence to evaluate the safety and effectiveness of vertebroplasty and kyphoplasty in cancer patients. -

Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy
Y Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines 1 G Evidence-Based Clinical Guidelines for Multidisciplinary ETHODOLO Spine Care M NE I DEL I U /G ON Diagnosis and Treatment of I NTRODUCT Lumbar Disc I Herniation with Radiculopathy NASS Evidence-Based Clinical Guidelines Committee D. Scott Kreiner, MD Paul Dougherty, II, DC Committee Chair, Natural History Chair Robert Fernand, MD Gary Ghiselli, MD Steven Hwang, MD Amgad S. Hanna, MD Diagnosis/Imaging Chair Tim Lamer, MD Anthony J. Lisi, DC John Easa, MD Daniel J. Mazanec, MD Medical/Interventional Treatment Chair Richard J. Meagher, MD Robert C. Nucci, MD Daniel K .Resnick, MD Rakesh D. Patel, MD Surgical Treatment Chair Jonathan N. Sembrano, MD Anil K. Sharma, MD Jamie Baisden, MD Jeffrey T. Summers, MD Shay Bess, MD Christopher K. Taleghani, MD Charles H. Cho, MD, MBA William L. Tontz, Jr., MD Michael J. DePalma, MD John F. Toton, MD This clinical guideline should not be construed as including all proper methods of care or excluding or other acceptable methods of care reason- ably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment is to be made by the physi- cian and patient in light of all circumstances presented by the patient and the needs and resources particular to the locality or institution. I NTRODUCT 2 Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines I ON Financial Statement This clinical guideline was developed and funded in its entirety by the North American Spine Society (NASS). All participating /G authors have disclosed potential conflicts of interest consistent with NASS’ disclosure policy.