This Article Appeared in a Journal Published by Elsevier. the Attached
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Fir Species in the Silviculture of British Forests
Kastamonu Üni., Orman Fakültesi Dergisi, 2012, Özel Sayı: 15-26 Kastamonu Univ., Journal of Forestry Faculty, 2012, Special Issue The Role of True Fir Species in the Silviculture of British Forests: past, present and future W.L. MASON Forest Research, Northern Research Station, Roslin, Midlothian, Scotland EH25 9SY, U.K. E.mail:[email protected] Abstract There are no true fir species (Abies spp.) native to the British Isles: the first to be introduced was Abies alba in the 1600s which was planted on some scale until the late 1800s when it proved vulnerable to an insect pest. Thereafter interest switched to North American species, particularly grand (Abies grandis) and noble (Abies procera) firs. Provenance tests were established for A. alba, A. amabilis, A. grandis, and A. procera. Other silver fir species were trialled in forest plots with varying success. Although species such as grand fir have proved highly productive on favourable sites, their initial slow growth on new planting sites and limited tolerance of the moist nutrient-poor soils characteristic of upland Britain restricted their use in the afforestation programmes of the last century. As a consequence, in 2010, there were about 8000 ha of Abies species in Britain, comprising less than one per cent of the forest area. Recent species trials have confirmed that best growth is on mineral soils and that, in open ground conditions, establishment takes longer than for other conifers. However, changes in forest policies increasingly favour the use of Continuous Cover Forestry and the shade tolerant nature of many fir species makes them candidates for use with selection or shelterwood silvicultural systems. -

Universidad Autónoma Agraria Antonio Narro
UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA Pinus arizonica Engelm. PRESENTA: SERGIO AMILCAR CANUL TUN MONOGRAFÍA Presentada como requisito parcial para Obtener el título de: INGENIERO FORESTAL Buenavista, Saltillo, Coahuila, México Mayo de 2005 UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA DEPARTAMENTO FORESTAL Pinus arizonica Engelm. MONOGRAFÍA Que somete a consideración del H. Jurado calificador como requisito parcial para obtener el título de: INGENIERO FORESTAL PRESENTA SERGIO AMILCAR CANUL TUN APROBADA PRESIDENTE DEL JURADO COORDINADOR DE LA DIVISIÓN DE AGRONOMÍA _____________________________ ________________________________ M. C. CELESTINO FLORES LÓPEZ M. C. ARNOLDO OYERVIDES GARCÍA Buenavista, Saltillo, Coahuila, México Mayo de 2005 UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA DEPARTAMENTO FORESTAL Pinus arizonica Engelm. MONOGRAFÍA Que somete a consideración del H. Jurado calificador como requisito parcial para obtener el título de: INGENIERO FORESTAL PRESENTA SERGIO AMILCAR CANUL TUN PRESIDENTE DEL JURADO _____________________________ M. C. CELESTINO FLORES LÓPEZ PRIMER SINODAL SEGUNDO SINODAL _______________________________ ___________________________________ Dr. MIGUEL ÁNGEL CAPÓ ARTEAGA Dr. JOSÉ ÁNGEL VILLARREAL QUINTANILLA Buenavista, Saltillo, Coahuila, México Mayo de 2005 DEDICATORIA A mis grandes amores: Leydi Abigail Cob Chan Nayvi Abisai Canul Cob Por haber llegado a mi vida y formar parte de mi, son un gran motivo para triunfar y salir adelante en medio de una gran tempestad. A mis padres: Ramón Cruz Canul Vázquez Lucila Tún Cahuich. Por su gran sacrificio realizado, por la confianza que me brindaron y por ser mis mejores amigos. Siempre tuve sus consejos, ayuda en el momento más crítico y comprensión. A mis hermanos: Claudio Ezequiel, por aceptar ser mi amigo, por su compañía y apoyo durante mi carrera y en este trabajo. -

Wa Shan – Emei Shan, a Further Comparison
photograph © Zhang Lin A rare view of Wa Shan almost minus its shroud of mist, viewed from the Abies fabri forested slopes of Emei Shan. At its far left the mist-filled Dadu River gorge drops to 500-600m. To its right the 3048m high peak of Mao Kou Shan climbed by Ernest Wilson on 3 July 1903. “As seen from the top of Mount Omei, it resembles a huge Noah’s Ark, broadside on, perched high up amongst the clouds” (Wilson 1913, describing Wa Shan floating in the proverbial ‘sea of clouds’). Wa Shan – Emei Shan, a further comparison CHRIS CALLAGHAN of the Australian Bicentennial Arboretum 72 updates his woody plants comparison of Wa Shan and its sister mountain, World Heritage-listed Emei Shan, finding Wa Shan to be deserving of recognition as one of the planet’s top hotspots for biological diversity. The founding fathers of modern day botany in China all trained at western institutions in Europe and America during the early decades of last century. In particular, a number of these eminent Chinese botanists, Qian Songshu (Prof. S. S. Chien), Hu Xiansu (Dr H. H. Hu of Metasequoia fame), Chen Huanyong (Prof. W. Y. Chun, lead author of Cathaya argyrophylla), Zhong Xinxuan (Prof. H. H. Chung) and Prof. Yung Chen, undertook their training at various institutions at Harvard University between 1916 and 1926 before returning home to estab- lish the initial Chinese botanical research institutions, initiate botanical exploration and create the earliest botanical gardens of China (Li 1944). It is not too much to expect that at least some of them would have had personal encounters with Ernest ‘Chinese’ Wilson who was stationed at the Arnold Arboretum of Harvard between 1910 and 1930 for the final 20 years of his life. -

Climate Change and Coffee Adaptation: Developing Dendroclimatological Records in the Southern Volcanic Chain of Guatemala
Climate Change and Coffee Adaptation: Developing Dendroclimatological Records In the Southern Volcanic Chain of Guatemala. LASG Field Study Awards 2015. Report of fieldwork activities. Diego Pons My main objective was to collect several dendrochronological samples from the southern slope of the volcanic chain in Guatemala in sites above 2,500 m ASL. I worked with increment core samples from Abies guatemalensis Rehder (Guatemalan Fir) in order to reconstruct the climatic signal of precipitation for the region. This species has proven to be sensitive to the late dry season rainfall and negatively correlated with growing season temperature as well as negatively correlated with eastern tropical Pacific SST anomalies. The absence of long-term instrumental records represents a challenge for coffee growers in the region in terms of understanding and adapting to climate changes. Coffee still represents one of the main sources of income to the Guatemalan economy and it has been historically affected by variability of precipitation patterns, leading to several coffee crises in the country. With the chronologies derived from the field trip partially covered with funds from LASG Field Study Award, I intend to improve the overall understanding of the climate variability of the region. Following my stated objectives, I spend about two months is Guatemala for the purpose of collecting dendrochronological samples from the southern slopes of Guatemala’s volcanic chain. I visited the forest known as “Kanchej” located in Cantel, Quetzaltenango (14°46'21.47"N 91°26'35.56"W) at 10,200 ft. Several samples were collected during an intense fieldtrip to these Guatemalan highlands within the Samalá River upper watershed (figure 1). -

A Case Study of the Vulnerable Abies Guatemalensis in Guatemala
Conservation through utilization: a case study of the Vulnerable Abies guatemalensis in Guatemala Uffe Strandby Andersen,JosE´ Pablo Prado CO´ rdova Ulrik BrA¨ uner Nielsen,Carsten Smith Olsen,Charlotte Nielsen Marten SØrensen and Johannes Kollmann Abstract Conservation through utilization is a controversial Introduction strategy that deserves more attention from researchers and practitioners. This case study focuses on Abies guatemalen- ocio-economic research has established that strict con- sis, a Vulnerable Mesoamerican conifer that is illegally used Sservation by suspension of the rights of local commu- for timber, shingles, charcoal and Christmas tree produc- nities to use forests is problematic in developing countries tion. Conservation of the remnant populations would pre- that have extensive and highly dispersed forest resources serve some unique montane forests, with concomitant and limited capacities for enforcement of legislation 1992 1999 benefits for local water supplies and prevention of landslides. (Oakerson, ; Enters & Anderson, ; Steins & 1999 2003 As a conservation tool we suggest establishment of additional Edwards, ; Edmunds & Wollenberg, ). Conserva- A. guatemalensis Christmas tree plantations. These could tion strategies are needed that work with local communities generate income for local farmers and help halt poaching to ensure they benefit from conservation measures. The from natural stands. So far, 51 such plantations have been concept of conservation through utilization applies this 2008 established in Guatemala -

2 ACUERDO Por El Que Se Da a Conocer La Lista De Especies Y Pobl
Miércoles 5 de marzo de 2014 DIARIO OFICIAL (Primera Sección) 2 ACUERDO por el que se da a conocer la lista de especies y poblaciones prioritarias para la conservación. Al margen un sello con el Escudo Nacional, que dice: Estados Unidos Mexicanos.- Secretaría de Medio Ambiente y Recursos Naturales. JUAN JOSÉ GUERRA ABUD, Secretario de Medio Ambiente y Recursos Naturales, con fundamento en los artículos 32 Bis fracciones I y II, de la Ley Orgánica de la Administración Pública Federal; 5o. fracciones I, XI, 79, fracciones I, II, III, de la Ley General del Equilibrio Ecológico y la Protección al Ambiente; 61 y 62, de la Ley General de Vida Silvestre, y 5, fracción XXV, del Reglamento Interior de la SEMARNAT, y CONSIDERANDO Que la SEMARNAT de acuerdo con el Artículo 61 de la Ley General de Vida Silvestre (LGVS), tiene como obligación elaborar y publicar la lista de especies prioritarias para la conservación, con el fin de promover el desarrollo de proyectos para su conservación y recuperación, y con ello la de ecosistemas, hábitat y especies con los que se encuentran asociadas. Dicha lista se debe elaborar conforme a las disposiciones estipuladas en el artículo 61 de la LGVS y observando el procedimiento establecido para tal efecto y se dará a conocer con la opinión previa del Consejo Técnico Consultivo Nacional para la Conservación y Aprovechamiento Sustentable de la Vida Silvestre (CONAVIS) (LGVS Art. 61 y 62). Que es de suma importancia la conservación de especies, toda vez que presenta ventajas complementarias al atender necesidades específicas para aquellas que se encuentran en alguna categoría de riesgo. -
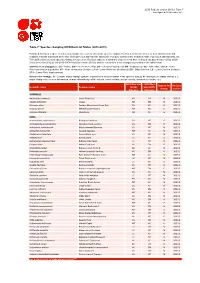
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Gabriel Eduardo Cervantes Angel Ingeniero Forestal
UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA DEPARTAMENTO FORESTAL Crecimiento de Pináceas Asociadas a Poblaciones Naturales de Picea mexicana Martínez en México Por: GABRIEL EDUARDO CERVANTES ANGEL TESIS Presentada como requisito parcial para obtener el título de: INGENIERO FORESTAL Saltillo, Coahuila, México. Diciembre, 2014 Este proyecto de tesis ha sido apoyado por el Proyecto de Investigación de la Universidad Autónoma Agraria Antonio Narro con No. 38-111-3613-2122, a cargo del profesor investigador Dr. Celestino Flores López. DEDICATORIA A Dios por ser un amigo y compañero, el motivo y guía en mi vida, todo fue posible gracias a su infinito amor, siempre te alabaré. A mis padres: Sra. Irma Angel Clemente Sr. Marcos Cervantes Molina. Quienes me dieron la vida, y procuraron darme alegría desde mi niñez. A ellos les agradezco la persona que hicieron de mí. A mi madre, por su incondicional apoyo en los momentos difíciles y por sus consejos oportunos. A mi padre, por sus regaños, consejos y la paciencia para enseñarme. Son mi orgullo, y siempre estaré agradecido con Dios por ser ustedes mis padres. Son la fortaleza y alegría de mi hogar. A mis hermanos y hermana: Sr. Eddy Ulises Cervantes Angel Sr. Marco Antonio Cervantes Angel Lic. Maricela Cervantes Angel. Quienes siempre han sido mis mejores amigos, en ustedes siempre encontré apoyo incondicional en los momentos difíciles de mi carrera y por su confianza que depositaron en mí. Son parte fundamental de mi vida y son parte de mi logro. A mi novia: Ing. Yocellyn Vázquez Ibarra. Por ser parte de mí vida, ser una persona comprensiva y apoyarme desde el inicio de nuestra hermosa relación, siempre tendrás un espacio muy especial en mi vida. -

Arceuthobium Abietinum
EPPO Datasheet: Arceuthobium abietinum Last updated: 2020-11-06 IDENTITY Preferred name: Arceuthobium abietinum Authority: (Engelmann) Engelmann ex Munz Taxonomic position: Plantae: Magnoliophyta: Angiospermae: Basal core eudicots: Santalales: Santalaceae Common names: fir dwarf mistletoe view more common names online... EPPO Categorization: A1 list view more categorizations online... EPPO Code: AREAB Notes on taxonomy and nomenclature Mathiasen and Kenaley (2019) have recombined the species Arceuthobium abietinum. The species consists of three subspecies, which are morphologically distinct and exhibit a high degree of host specificity: (1) Arceuthobium abietinum subsp. abietinum (formerly f.sp. concoloris) (white fir dwarf mistletoe), (2) Arceuthobium abietinum subsp. magnificae (formerly f.sp. magnificae) (red fir dwarf mistletoe), (3) Arceuthobium abietinum subsp. wiensii (Wiens' dwarf mistletoe). The new designations from Mathiasen and Kenaley (2019) are used in the datasheet. Recently, Kenaley (2020) proposed two further subspecies, namely Arceuthobium abietinum subsp. mathiasenii (Mathiasen’s dwarf mistletoe), and Arceuthobium abietinum subsp. grandae (grand fir dwarf mistletoe). HOSTS Arceuthobium abietinum subsp. abietinum is a common plant parasite of Abies grandis (Douglas ex D. Don) Lindl. (grand fir), Abies lowiana (Gordon) A. Murray bis (Sierra white fir), Abies concolor (Gord. et Glend.) Lindl. ex Hildebr. (Rocky Mountain white fir), and the hybrid populations of Abies concolor x Abies grandis in the Pacific Northwest and California, USA (Hawksworth et al., 2002; Mathiasen, 2019; Mathiasen and Kenaley, 2019). It also parasitizes Abies durangensis Martínez (Durango fir) in Durango and Chihuahua, Mexico (Mathiasen, 2010; Quiñonez Barraza et al., 2013) and produces heavy infections on Picea breweriana Watson (Brewer spruce) (Hawksworth et al., 2002) Arceuthobium abietinum subsp. -

Mistletoes of North American Conifers
United States Department of Agriculture Mistletoes of North Forest Service Rocky Mountain Research Station American Conifers General Technical Report RMRS-GTR-98 September 2002 Canadian Forest Service Department of Natural Resources Canada Sanidad Forestal SEMARNAT Mexico Abstract _________________________________________________________ Geils, Brian W.; Cibrián Tovar, Jose; Moody, Benjamin, tech. coords. 2002. Mistletoes of North American Conifers. Gen. Tech. Rep. RMRS–GTR–98. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 123 p. Mistletoes of the families Loranthaceae and Viscaceae are the most important vascular plant parasites of conifers in Canada, the United States, and Mexico. Species of the genera Psittacanthus, Phoradendron, and Arceuthobium cause the greatest economic and ecological impacts. These shrubby, aerial parasites produce either showy or cryptic flowers; they are dispersed by birds or explosive fruits. Mistletoes are obligate parasites, dependent on their host for water, nutrients, and some or most of their carbohydrates. Pathogenic effects on the host include deformation of the infected stem, growth loss, increased susceptibility to other disease agents or insects, and reduced longevity. The presence of mistletoe plants, and the brooms and tree mortality caused by them, have significant ecological and economic effects in heavily infested forest stands and recreation areas. These effects may be either beneficial or detrimental depending on management objectives. Assessment concepts and procedures are available. Biological, chemical, and cultural control methods exist and are being developed to better manage mistletoe populations for resource protection and production. Keywords: leafy mistletoe, true mistletoe, dwarf mistletoe, forest pathology, life history, silviculture, forest management Technical Coordinators_______________________________ Brian W. Geils is a Research Plant Pathologist with the Rocky Mountain Research Station in Flagstaff, AZ. -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

CITES and Timber (PDF)
This guide covers the main timber species regulated CITES and Timber by the Convention on International Trade in Endangered Species (CITES). It provides information CITES and Timber on the key issues regarding the implementation of the Convention for this important group of plants. A guide to CITES-listed tree species Written for the non-expert, individual sections cover the species found in significant trade, with details on their distribution, uses, traded parts and derivatives, and scientific and common names. Madeleine Groves Madeleine Groves Additional sections cover timber identification and measurement, guidance on CITES documentation and key resources. and Catherine Rutherford shop.kew.org/kewbooksonline Madeleine Groves Catherine Rutherford CITES and Timber A guide to CITES-listed tree species Madeleine Groves Catherine Rutherford © The Board of Trustees of the Royal Botanic Gardens, Kew 2015 Illustrations and photographs © Royal Botanic Gardens, Kew, unless otherwise stated in the captions The authors have asserted their rights to be identified as the authors of this work in accordance with the Copyright, Designs and Patents Act 1988 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form, or by any means, electronic, mechanical, photocopying, recording or otherwise, without written permission of the publisher unless in accordance with the provisions of the Copyright Designs and Patents Act 1988. Great care has been taken to maintain the accuracy of the information contained in this work. However, neither the publisher, the editors nor authors can be held responsible for any consequences arising from use of the information contained herein.