Extracellular Matrix in Vascular Morphogenesis and Disease: Structure Versus Signalq
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Basic Histology and Connective Tissue Chapter 5
Basic Histology and Connective Tissue Chapter 5 • Histology, the Study of Tissues • Tissue Types • Connective Tissues Histology is the Study of Tissues • 200 different types of cells in the human body. • A Tissue consist of two or more types of cells that function together. • Four basic types of tissues: – epithelial tissue – connective tissue – muscular tissue – nervous tissue • An Organ is a structure with discrete boundaries that is composed of 2 or more tissue types. • Example: skin is an organ composed of epidermal tissue and dermal tissue. Distinguishing Features of Tissue Types • Types of cells (shapes and functions) • Arrangement of cells • Characteristics of the Extracellular Matrix: – proportion of water – types of fibrous proteins – composition of the ground substance • ground substance is the gelatinous material between cells in addition to the water and fibrous proteins • ground substance consistency may be liquid (plasma), rubbery (cartilage), stony (bone), elastic (tendon) • Amount of space occupied by cells versus extracellular matrix distinguishes connective tissue from other tissues – cells of connective tissues are widely separated by a large amount of extracellular matrix – very little extracellular matrix between the cells of epithelia, nerve, and muscle tissue Embryonic Tissues • An embryo begins as a single cell that divides into many cells that eventually forms 3 Primary Layers: – ectoderm (outer layer) • forms epidermis and nervous system – endoderm (inner layer) • forms digestive glands and the mucous membrane lining digestive tract and respiratory system – mesoderm (middle layer) • Forms muscle, bone, blood and other organs. Histotechnology • Preparation of specimens for histology: – preserve tissue in a fixative to prevent decay (formalin) – dehydrate in solvents like alcohol and xylene – embed in wax or plastic – slice into very thin sections only 1 or 2 cells thick – float slices on water and mount on slides and then add color with stains • Sectioning an organ or tissue reduces a 3-dimensional structure to a 2- dimensional slice. -

Connective Tissue • Includes Things Like Bone, Fat, & Blood. All
Connective Tissue • includes things like bone, fat, & blood. All connective tissues include: 1. specialized cells 2.extracellular protein fibers } matrix that surrounds cells. 3. a fluid known as ground substance Functions include: Connective tissues come in 3 major types •Establish a structural framework 1. Connective tissue proper •Transporting fluids from one part of the body to another 2. Fluid Connective Tissue •Protecting delicate organs •Supporting, surrounding and interconnecting 3. Supporting Connective Tissue other tissue types • Other CTP cells are involved in defense and Connective Tissue Proper large repair jobs (these roam from site to site as • Connective tissue with many cell types and needed) extracellular fibers in a syrupy ground substance. A. Macrophages • Some cells of CTP are involved w/repair, B. Mast cells maintenance, and energy storage. C. Lymphocytes a. Fibroblasts D. plasma cells E. Microphages b. Adipocytes • The number of cells and cell types within a tissue at c. Mesenchymal cells any given moment varies depending on local conditions. 1 The Cell Population C. Adipocytes A. Fibroblasts • Fat cells • Most abundant cells in CTP • Typically contain a single enormous lipid droplet • Permanent resident of CTP (always present) • Other organelles squeezed to side of cell wall • Produce proteins to make the ground substance (resemble a class ring) very viscous • Also secret e prot ei ns th at mak e th e fib ers DMD. Mesenc hyma l ce lls • Stem cells B. Macrophages • Large amoeboid cells • Respond to injury by dividing into daughter cells which differentiate into connective tissue cells • Engulf & digest pathogens or damaged cells that enter the tissue • Release chemicals that activate the bodies immune system E. -

Connective Tissue N. Swailes, Ph.D. Department of Anatomy and Cell
Module 1.3: Connective Tissue N. Swailes, Ph.D. Department of Anatomy and Cell Biology Rm: B046A ML Tel: 5-7726 E-mail: [email protected] Required reading Mescher AL, Junqueira’s Basic Histology Text and Atlas, 13th Edition, Chapter 5 (also via AccessMedicine) Learning objectives 1) Name the three major classes of connective tissue and give examples of each. 2) Identify and describe the origin, organization and fate of embryonic connective tissue 3) Identify and discuss the functional properties imparted to tissue by the extracellular matrix: a. fibers (elastin, collagen Type I, II, III, IV and VII) b. ground substance (glycosaminoglycans, proteoglycans, glycoproteins) 4) Distinguish between different connective tissue cells and discuss their roles: a. fibroblasts b. adipocytes c. macrophages d. mast cells e. lymphocytes f. plasma cells g. eosinophils h. neutrophils 5) Classify the different connective tissues proper and compare and contrast their functional roles within an organ. Introduction The human body is made up of only four basic tissues: 1. Epithelial tissue 2. Connective tissue 3. Muscle tissue 4. Nervous tissue By adjusting the organization, composition and special features associated with each of these tissues is is possible to impart a wide variety of functions to the region or organ that they form. During this lecture you will examine the basic histological structure and function of Connective Tissue. 1 | Page: Connective Tissue Swailes a loose meshwork Part A: General characteristics of connective tissues that cushions and allows diffusion A1. There are three major classes of connective tissue i. Connective tissues proper - the most common class of connective tissue in the body. -

Pg 131 Chondroblast -> Chondrocyte (Lacunae) Firm Ground Substance
Figure 4.8g Connective tissues. Chondroblast ‐> Chondrocyte (Lacunae) Firm ground substance (chondroitin sulfate and water) Collagenous and elastic fibers (g) Cartilage: hyaline No BV or nerves Description: Amorphous but firm Perichondrium (dense irregular) matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. Matrix Costal Photomicrograph: Hyaline cartilage from the cartilages trachea (750x). Thickness? Metabolism? Copyright © 2010 Pearson Education, Inc. Pg 131 Figure 6.1 The bones and cartilages of the human skeleton. Epiglottis Support Thyroid Larynx Smooth Cartilage in Cartilages in cartilage external ear nose surface Cricoid Trachea Articular Lung Cushions cartilage Cartilage of a joint Cartilage in Costal Intervertebral cartilage disc Respiratory tube cartilages in neck and thorax Pubic Bones of skeleton symphysis Meniscus (padlike Axial skeleton cartilage in Appendicular skeleton knee joint) Cartilages Articular cartilage of a joint Hyaline cartilages Elastic cartilages Fibrocartilages Pg 174 Copyright © 2010 Pearson Education, Inc. Figure 4.8g Connective tissues. (g) Cartilage: hyaline Description: Amorphous but firm matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. -

Mbios 401: Lecture 10.2 Extracellular Matrix Slide #1 Extracellular Matrix
MBios 401: Lecture 10.2 Extracellular Matrix Slide #1 Extracellular Matrix Shelden, Chapter 19, Basal Lamina. Audio: The extracellular matrix is the only topic in this course where we’ll be discussing something that’s fundamentally not a cell or part of a cell. Instead, the extracellular matrix, or ECM, is mostly made up of a material known as ground substance, along with a variety of types of imbedded protein fibers. Cells are the third and least abundant component of the extracellular matrix. However, since the extracellular matrix is made by cells and plays a significant role in determining the behavior of cells in it, we’ll discuss the cells of the extracellular matrix briefly. Every distinct tissue in an organism expresses characteristic extracellular matrix components, which gives the tissue its physical characteristics and also give the cells imbedded in it signals that they have to interpret correctly. As a consequence, the composition of the extracellular matrix is highly variable, and we’ll be looking at some of the molecular mechanisms that give rise to this variability. However, there are only two major types of extracellular matrix materials, which are shown in the cartoon image at the top of this slide. Most of this picture is filled with the first type, which includes the grey shaded material and the red and green imbedded fibers. This tissue, which is called connective tissue, forms most of the extracellular matrix material in our bodies. It has a variety of roles, including the formation of conduits for nerves and blood cells. It also provides supporting material for cellular organs, such as the liver and kidney and it performs a variety of mechanical roles in our bodies, including the formation of the skeletal system and things like tendons and ligaments. -

Connective Tissue 2
Tissue Physiology Connective tissue is one of the most abundant and widely distributed tissues in the body. It forms our bones, surrounds our organs, allows for the integrity of our neural and vascular system, cushions and lubricates our joints, and connects the muscles to our skeleton. Connective tissue serves multiple functions. It provides the organism with shape and mechanical support. Also, it modulates cell migration, growth, and differentiation. The following are major features of connective tissue: structural and mechanical, defense, nutrition and transport of molecules and storage. Connective tissue supports the shape of cells, tissues, and organs interacting with the cytoskeleton. The most obvious structural connective tissue is bone, comprising the skeleton and supporting the entire organism. It contains cells and metabolites important in immune function, such as inflammation, and in tissue repair after injury. Blood and blood vessels are connective tissue, which transports substances throughout the body. The nervous system is housed within connective tissue. Components in connective tissue regulate movement of nutrients between cells. Adipose tissue is a unique connective tissue, providing storage of energy and insulation. Connective tissue function is mediated by its different components, most of which are macromolecules that interact with one another and with the cells. Varying the proportions and the arrangement of the individual components determines the function of the particular tissue. Nutrient deficiencies may disrupt regulation of tissue synthesis and degradation. Connective tissue is metabolically active and serves many functions. The tissue consists of three basic components: fibers, ground substance, and cells. Outside the cell the fibers and ground substance form the extra cellular matrix. -

Chapter 2 Extracellular Matrix and Cardiac Remodeling
Chapter 2 Extracellular Matrix and Cardiac Remodeling Bodh I. Jugdutt University of Alberta‚ Edmonton‚ Alberta 1. Introduction Cumulative evidence over the last 3 decades suggests that the cardiac interstitium or extracellular matrix (ECM)‚ especially the extracellular collagen matrix (ECCM)‚ plays an important role in cardiac remodeling during various cardiac diseases including myocardial infarction (MI) and heart failure [1-4]. A unique feature of the healthy heart is the ability of the pumping chambers to return to the ideal shape at end-diastole despite repetitive changes during every cardiac cycle throughout life. This physiologic remodeling or property of resuming functional shape depends largely on the integrity of the ECCM‚ the intricate network of fibrillar collagens found in the ECM. In a broad sense‚ pathophysiologic cardiac remodeling refers to changes in cardiac structure‚ shape and function following stress or damage [5-7]. To remodel is defined in the Webster dictionary as “ to alter the structure‚ to remake” and in the Oxford dictionary as “to model again and differently‚ reconstruct and reorganize.” The Oxford dictionary defines “model” as “representation in three dimensions of the proposed structure” and “to model” as “to fashion and shape.” This review will focus on the role of the ECCM in pathologic cardiac remodeling and specifically left ventricular (LV) remodeling. 2. Cardiac extracellular matrix 2.1. Cardiac ECM‚ ECCM and fibroblasts In the heart‚ as in other organs [8‚ 9]‚ the specialized parenchymal cells (cardiomyocytes) are supported by an ECM made up of an intricate macromolecular network of fibers and different cell types of mesenchymal origin including fibroblasts‚ endothelial cells (cardiac and vascular)‚ smooth 24 muscle cells‚ blood-borne cells (macrophages and others)‚ pericytes and neurons‚ bathed in a gel-type ground substance (Table 1). -
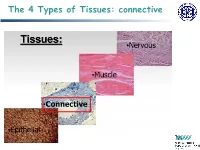
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

Ground Substance of the Connective Tissues A
Postgrad Med J: first published as 10.1136/pgmj.41.477.382 on 1 July 1965. Downloaded from POSTGRAD. MED. J. (1965), 41, 382 RECENT TRENDS IN THE BIOCHEMISTRY OF THE GROUND SUBSTANCE OF THE CONNECTIVE TISSUES A. G. LLOYD, B.Sc., Ph.D., A.R.I.C. Senior Lecturer in Biochemistry and Member of the M.R.C. Research Group for Studies on Connective Tissue and Lung, University College, Cardiff. THE UBIQUITY of members of the anatomical to the surrounding tissue, the fibres usually group loosely defined as connective tissues is being described as collagenous. As yet there a matter of long-standing record. The same is no clear indication whether such fibroses comment applies to the convention that, despite arise according to the partially established the differing biological roles which they are pattern of inflammatory response in which the required to play and their characteristically earliest recognisable biochemical changes in- different mechanical character at macroscopic clude alterations in the ground substance of level, the individual members of the connective connective tissues followed only subsequently tissue group exhibit certain basic similarities by de novo formation of additional fibrous which first become apparent at microscopic material. It is for this reason that the research level. A common biochemical approach is to group housed in the Department of Biochemis- develop these features of similarity to a point try is designed to assist in the clarification where an idealised composite of all connective of fundamental biochemical changes associated tissues can be devised. Use of such a model with general lung dust diseases from the aspect allows initial broad generalisations to be drawn. -

Mast Cells of the Omentum in Relation to States of Adrenocortical Deficiency and Excess*
MAST CELLS OF THE OMENTUM IN RELATION TO STATES OF ADRENOCORTICAL DEFICIENCY AND EXCESS* By Burton L. Baker Department oj Anatomy, liniversity oj Michigan Medical School, Ann Arbor, Michigan Little is known of the function of several components of loose fibroelastic connective tissue, the mast cells, in particular, having been resistant to experi- mental attack. Nevertheless, a discussion of them is appropriate to a confer- ence on mechanisms of adrenocortical hormone action for several reasons. First, it is generally agreed that mast cells contain a mucopolysaccharide that, according to LisonL6and Holmgren and Wilander,14 stains metachromatically because of its esterification with sulfuric acid, the latter investigators conclud- ing that this substance is heparin. Asboe-Hansen3 reports that injection of corticotropin into man causes a marked reduction in the number of mast cells. Others claim that the coagulation time may be reducedLnor prolonged, with an increase in the quantity of circulating heparin-like substances,'* during treat- ment of human patients with corticotropin or cortisone. These effects require further study, however, because of the wide variation in the values obtained by the technical methods employed.12 Second, other workers believe that mast cells produce the mucopolysaccharide hyaluronic acid, which is an important constituent of the ground substance in connective tissue. Although not all histologists share this viewpoint, consid- erable inferential evidence may be marshalled to support it. Mast cells accu- mulate in the dermis bordering areas of epidermal hyperplasia resulting from applications of carcinogens to mouse skin. Cramer and Simpson" regard this occurrence as a possible protective action on the part of mast cells to supple- ment the hyaluronic acid of the dermal ground substance. -
Connective Tissue – Material Found Between Cells – Supports and Binds Structures Together – Stores Energy As Fat – Provides Immunity to Disease
Chapter 4 The Tissue Level of Organization • Group of similar cells – common function • Histology – study of tissues • Pathologist – looks for tissue changes that indicate disease 4-1 4 Basic Tissues (1) • Epithelial Tissue – covers surfaces because cells are in contact – lines hollow organs, cavities and ducts – forms glands when cells sink under the surface • Connective Tissue – material found between cells – supports and binds structures together – stores energy as fat – provides immunity to disease 4-2 4 Basic Tissues (2) • Muscle Tissue – cells shorten in length producing movement • Nerve Tissue – cells that conduct electrical signals – detects changes inside and outside the body – responds with nerve impulses 4-3 Epithelial Tissue -- General Features • Closely packed cells forming continuous sheets • Cells sit on basement membrane • Apical (upper) free surface • Avascular---without blood vessels – nutrients diffuse in from underlying connective tissue • Rapid cell division • Covering / lining versus glandular types 4-4 Basement Membrane • holds cells to connective tissue 4-5 Types of Epithelium • Covering and lining epithelium – epidermis of skin – lining of blood vessels and ducts – lining respiratory, reproductive, urinary & GI tract • Glandular epithelium – secreting portion of glands – thyroid, adrenal, and sweat glands 4-6 Classification of Epithelium • Classified by arrangement of cells into layers – simple = one cell layer thick – stratified = many cell layers thick – pseudostratified = single layer of cells where all cells -

A Test of Connective Tissue State and Reactivity in Collagen Diseases
A TEST OF CONNECTIVE TISSUE STATE AND REACTIVITY IN COLLAGEN DISEASES Daniel M. Laskin, … , Norman R. Joseph, Victor E. Pollak J Clin Invest. 1961;40(12):2153-2161. https://doi.org/10.1172/JCI104441. Research Article Find the latest version: https://jci.me/104441/pdf A TEST OF CONNECTIVE TISSUE STATE AND REACTIVITY IN COLLAGEN DISEASES * By DANIEL M. LASKIN, MILTON B. ENGEL, NORMAN R. JOSEPH AND VICTOR E. POLLAK t (From the Colleges of Dentistry, Pharmacy and Medicine, University of Illinois, Chicago, Ill.) (Submitted for publication January 12, 1961; accepted August 17, 1961) In the group of conditions known as "collagen bears a net negative charge; this is distributed be- diseases" there are often morphological changes tween the two phases. The colloidal charge gov- in discrete areas of the connective tissue. For ex- erns the distribution of mobile cations and anions ample, fibrinoid degeneration of the collagen fibers through thermodynamic and electrostatic equilib- and changes in the ground substance may be seen rium and through the chemical binding of ions to in the subcutaneous nodules of rheumatoid arthri- the matrix. Electrolyte concentrations and water tis and in the skin lesions of lupus erythematosus. content in connective tissues are thus determined These lesions are not well understood. The more by the colloidal structure (1-5). generalized alterations in the connective tissue Collagen, glycoproteins and mucopolysaccha- are even more difficult to demonstrate and remain rides, including chondroitin sulfate and hyalu- poorly defined. Using an in vivo electrometric ronic acid, have been conceived to be constituents method, we have previously determined certain of a complex coacervate in the matrix of dermal physicochemical properties of normal mammalian connective tissue (12).