Element Entry Identification What Is the Atomic Number? Terms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
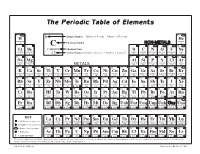
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

Power Sources Challenge
POWER SOURCES CHALLENGE FUSION PHYSICS! A CLEAN ENERGY Summary: What if we could harness the power of the Sun for energy here on Fusion reactions occur when two nuclei come together to form one Earth? What would it take to accomplish this feat? Is it possible? atom. The reaction that happens in the sun fuses two Hydrogen atoms together to produce Helium. It looks like this in a very simplified way: Many researchers including our Department of Energy scientists and H + H He + ENERGY. This energy can be calculated by the famous engineers are taking on this challenge! In fact, there is one DOE Laboratory Einstein equation, E = mc2. devoted to fusion physics and is committed to being at the forefront of the science of magnetic fusion energy. Each of the colliding hydrogen atoms is a different isotope of In order to understand a little more about fusion energy, you will learn about hydrogen, one deuterium and one the atom and how reactions at the atomic level produce energy. tritium. The difference in these isotopes is simply one neutron. Background: It all starts with plasma! If you need to learn more about plasma Deuterium has one proton and one physics, visit the Power Sources Challenge plasma activities. neutron, tritium has one proton and two neutrons. Look at the The Fusion Reaction that happens in the SUN looks like this: illustration—do you see how the mass of the products is less than the mass of the reactants? That is called a mass deficit and that difference in mass is converted into energy. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

No. It's Livermorium!
in your element Uuh? No. It’s livermorium! Alpha decay into flerovium? It must be Lv, saysKat Day, as she tells us how little we know about element 116. t the end of last year, the International behaviour in polonium, which we’d expect to Union of Pure and Applied Chemistry have very similar chemistry. The most stable A(IUPAC) announced the verification class of polonium compounds are polonides, of the discoveries of four new chemical for example Na2Po (ref. 8), so in theory elements, 113, 115, 117 and 118, thus Na2Lv and its analogues should be attainable, completing period 7 of the periodic table1. though they are yet to be synthesized. Though now named2 (no doubt after having Experiments carried out in 2011 showed 3 213 212m read the Sceptical Chymist blog post ), that the hydrides BiH3 and PoH2 were 9 we shall wait until the public consultation surprisingly thermally stable . LvH2 would period is over before In Your Element visits be expected to be less stable than the much these ephemeral entities. lighter polonium hydride, but its chemical In the meantime, what do we know of investigation might be possible in the gas their close neighbour, element 116? Well, after phase, if a sufficiently stable isotope can a false start4, the element was first legitimately be found. reported in 2000 by a collaborative team Despite the considerable challenges posed following experiments at the Joint Institute for by the short-lived nature of livermorium, EMMA SOFIA KARLSSON, STOCKHOLM, SWEDEN STOCKHOLM, KARLSSON, EMMA SOFIA Nuclear Research (JINR) in Dubna, Russia. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Hydrogen and Its Components
HYDROGEN AND ITS COMPONENTS Discovery In 1671, Robert Boyle discovered and described the reaction between iron filings and dilute acids, which resulted in the production of hydrogen gas. In 1766-81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produced water when burned. He named it "flammable air". In 1783, Antoine Lavoisier gave the element the name hydrogen (from the Greek σδρο-hydro meaning "water" and -γενης genes meaning "creator") when he and Pierre-Simon Laplace reproduced Cavendish's finding that water was produced when hydrogen was burned. Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling and his invention, the vacuum flask. He produced solid hydrogen the next year. Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck. Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932. The nickel hydrogen battery was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2). It had two caesium atomic clocks on board and helped to show that satellite navigation based on precise timing was possible. In the dark part of its orbit, the Hubble Space Telescope is powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch, and 13 years passed their design life. from NASA (accessed 2 Feb 2015) Isotopes of hydrogen Hydrogen has three naturally occurring isotopes, denoted 1H, 2H and 3H. -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Walter C. Ermler
Curriculum Vitae Dr. Walter C. Ermler Professor of Chemistry Department of Chemistry, BSE-1.104E University of Texas at San Antonio One UTSA Circle San Antonio, TX 78249-0698 Phone: 210-458-7005 Fax: 210-458-7428 Email: [email protected] Homepage: https://chemistry.utsa.edu/archives/831 Education Doctor of Philosophy (Physical Chemistry), Ohio State University, 1972 Master of Science (Physical Chemistry), Ohio State University, 1970 Bachelor of Science (Chemistry), Northern Illinois University, 1969 Professional Employment History Academic: 1978-present Professor of Chemistry, University of Texas at San Antonio, 2005-present Chair, Department of Chemistry, University of Texas at San Antonio, 2005-2006 Professor of Physics, Stevens Institute of Technology, 1990-2001 Chair, Dept. Chemistry and Chemical Biology, Stevens Institute of Technology, 1998-1999 Professor of Chemistry, Stevens Institute of Technology, 1984-2001 Associate Professor of Chemistry, Stevens Institute of Technology, 1978-83 Federal: 1993-2019 Program Director, National Science Foundation, MPS-CHE-CTMC/REU, 2018-2020 Program Director, National Science Foundation, MPS-CHE, EHR-REC, 2001-2005 Senior Program Manager, U. S. Department of Energy, ASCR, 1993-1996 Postdoctoral Research Associate: 1973-1978 University of California, Berkeley, Department of Chemistry, 1976-1978 (Kenneth S. Pitzer, Priestly Medalist) University of Chicago Departments of Physics and Chemistry, 1973-1976 (Robert S. Mulliken, Nobel Laureate) Awards and Honors Wiley Visiting Scientist Fellow, EMSL, -

The Isotope Effect: Prediction, Discussion, and Discovery
1 The isotope effect: Prediction, discussion, and discovery Helge Kragh Centre for Science Studies, Department of Physics and Astronomy, Aarhus University, 8000 Aarhus, Denmark. ABSTRACT The precise position of a spectral line emitted by an atomic system depends on the mass of the atomic nucleus and is therefore different for isotopes belonging to the same element. The possible presence of an isotope effect followed from Bohr’s atomic theory of 1913, but it took several years before it was confirmed experimentally. Its early history involves the childhood not only of the quantum atom, but also of the concept of isotopy. Bohr’s prediction of the isotope effect was apparently at odds with early attempts to distinguish between isotopes by means of their optical spectra. However, in 1920 the effect was discovered in HCl molecules, which gave rise to a fruitful development in molecular spectroscopy. The first detection of an atomic isotope effect was no less important, as it was by this means that the heavy hydrogen isotope deuterium was discovered in 1932. The early development of isotope spectroscopy illustrates the complex relationship between theory and experiment, and is also instructive with regard to the concepts of prediction and discovery. Keywords: isotopes; spectroscopy; Bohr model; atomic theory; deuterium. 1. Introduction The wavelength of a spectral line arising from an excited atom or molecule depends slightly on the isotopic composition, hence on the mass, of the atomic system. The phenomenon is often called the “isotope effect,” although E-mail: [email protected]. 2 the name is also used in other meanings. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Critical Mineral Resources of the United States— Economic and Environmental Geology and Prospects for Future Supply
Critical Mineral Resources of the United States— Economic and Environmental Geology and Prospects for Future Supply Professional Paper 1802 U.S. Department of the Interior U.S. Geological Survey Periodic Table of Elements 1A 8A 1 2 hydrogen helium 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 lithium beryllium boron carbon nitrogen oxygen fluorine neon 6.94 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sodium magnesium aluminum silicon phosphorus sulfur chlorine argon 22.99 24.31 3B 4B 5B 6B 7B 8B 11B 12B 26.98 28.09 30.97 32.06 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.64 74.92 78.96 79.90 83.79 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium indium tin antimony tellurium iodine xenon 85.47 87.62 88.91 91.22 92.91 95.96 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 cesium barium hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon 132.9 137.3 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.5 204.4 207.2 209.0 (209) (210)(222) 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 francium radium rutherfordium -

BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged To
BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged to Four and Five Significant Figures Norman E. Holden Energy Sciences & Technology Department National Nuclear Data Center Brookhaven National Laboratory P.O. Box 5000 Upton, NY 11973-5000 www.bnl.gov Prepared for the 44th IUPAC General Assembly, in Torino, Italy August 2007 Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CH10886 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. This preprint is intended for publication in a journal or proceedings. Since changes may be made before publication, it may not be cited or reproduced without the author’s permission. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors.