Co-Existence of the Double Inferior Vena Cava with Complex Interiliac
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prep for Practical II
Images for Practical II BSC 2086L "Endocrine" A A B C A. Hypothalamus B. Pineal Gland (Body) C. Pituitary Gland "Endocrine" 1.Thyroid 2.Adrenal Gland 3.Pancreas "The Pancreas" "The Adrenal Glands" "The Ovary" "The Testes" Erythrocyte Neutrophil Eosinophil Basophil Lymphocyte Monocyte Platelet Figure 29-3 Photomicrograph of a human blood smear stained with Wright’s stain (765). Eosinophil Lymphocyte Monocyte Platelets Neutrophils Erythrocytes "Blood Typing" "Heart Coronal" 1.Right Atrium 3 4 2.Superior Vena Cava 5 2 3.Aortic Arch 6 4.Pulmonary Trunk 1 5.Left Atrium 12 9 6.Bicuspid Valve 10 7.Interventricular Septum 11 8.Apex of The Heart 9. Chordae tendineae 10.Papillary Muscle 7 11.Tricuspid Valve 12. Fossa Ovalis "Heart Coronal Section" Coronal Section of the Heart to show valves 1. Bicuspid 2. Pulmonary Semilunar 3. Tricuspid 4. Aortic Semilunar 5. Left Ventricle 6. Right Ventricle "Heart Coronal" 1.Pulmonary trunk 2.Right Atrium 3.Tricuspid Valve 4.Pulmonary Semilunar Valve 5.Myocardium 6.Interventricular Septum 7.Trabeculae Carneae 8.Papillary Muscle 9.Chordae Tendineae 10.Bicuspid Valve "Heart Anterior" 1. Brachiocephalic Artery 2. Left Common Carotid Artery 3. Ligamentum Arteriosum 4. Left Coronary Artery 5. Circumflex Artery 6. Great Cardiac Vein 7. Myocardium 8. Apex of The Heart 9. Pericardium (Visceral) 10. Right Coronary Artery 11. Auricle of Right Atrium 12. Pulmonary Trunk 13. Superior Vena Cava 14. Aortic Arch 15. Brachiocephalic vein "Heart Posterolateral" 1. Left Brachiocephalic vein 2. Right Brachiocephalic vein 3. Brachiocephalic Artery 4. Left Common Carotid Artery 5. Left Subclavian Artery 6. Aortic Arch 7. -

Corona Mortis: the Abnormal Obturator Vessels in Filipino Cadavers
ORIGINAL ARTICLE Corona Mortis: the Abnormal Obturator Vessels in Filipino Cadavers Imelda A. Luna Department of Anatomy, College of Medicine, University of the Philippines Manila ABSTRACT Objectives. This is a descriptive study to determine the origin of abnormal obturator arteries, the drainage of abnormal obturator veins, and if any anastomoses exist between these abnormal vessels in Filipino cadavers. Methods. A total of 54 cadaver halves, 50 dissected by UP medical students and 4 by UP Dentistry students were included in this survey. Results. Results showed the abnormal obturator arteries arising from the inferior epigastric arteries in 7 halves (12.96%) and the abnormal communicating veins draining into the inferior epigastric or external iliac veins in 16 (29.62%). There were also arterial anastomoses in 5 (9.25%) with the inferior epigastric artery, and venous anastomoses in 16 (29.62%) with the inferior epigastric or external iliac veins. Bilateral abnormalities were noted in a total 6 cadavers, 3 with both arterial and venous, and the remaining 3 with only venous anastomoses. Conclusion. It is important to be aware of the presence of these abnormalities that if found during surgery, must first be ligated to avoid intraoperative bleeding complications. Key Words: obturator vessels, abnormal, corona mortis INtroDUCTION The main artery to the pelvic region is the internal iliac artery (IIA) with two exceptions: the ovarian/testicular artery arises directly from the aorta and the superior rectal artery from the inferior mesenteric artery (IMA). The internal iliac or hypogastric artery is one of the most variable arterial systems of the human body, its parietal branches, particularly the obturator artery (OBA) accounts for most of its variability. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Cat Dissection
Cat Dissection Muscular Labs Tibialis anterior External oblique Pectroalis minor Sartorius Gastrocnemius Pectoralis major Levator scapula External oblique Trapezius Gastrocnemius Semitendinosis Trapezius Latissimus dorsi Sartorius Gluteal muscles Biceps femoris Deltoid Trapezius Deltoid Lumbodorsal fascia Sternohyoid Sternomastoid Pectoralis minor Pectoralis major Rectus abdominis Transverse abdominis External oblique External oblique (reflected) Internal oblique Lumbodorsal Deltoid fascia Latissimus dorsi Trapezius Trapezius Trapezius Deltoid Levator scapula Deltoid Trapezius Trapezius Trapezius Latissimus dorsi Flexor carpi radialis Brachioradialis Extensor carpi radialis Flexor carpi ulnaris Biceps brachii Triceps brachii Biceps brachii Flexor carpi radialis Flexor carpi ulnaris Extensor carpi ulnaris Triceps brachii Extensor carpi radialis longus Triceps brachii Deltoid Deltoid Deltoid Trapezius Sartorius Adductor longus Adductor femoris Semimembranosus Vastus Tensor fasciae latae medialis Rectus femoris Vastus lateralis Tibialis anterior Gastrocnemius Flexor digitorum longus Biceps femoris Tensor fasciae latae Semimembranosus Semitendinosus Gluteus medius Gluteus maximus Extensor digitorum longus Gastrocnemius Soleus Fibularis muscles Brachioradiallis Triceps (lateral and long heads) Brachioradialis Biceps brachii Triceps (medial head) Trapezius Deltoid Deltoid Levator scapula Trapezius Deltoid Trapezius Latissimus dorsi External oblique (right side cut and reflected) Rectus abdominis Transversus abdominis Internal oblique Pectoralis -

Variant Branching Pattern of the Right Internal Iliac Vessels in a Male
Case Report Original Article Archives of Clinical Experimental Surgery Increased of Langerhans Cells in Smokeless Tobacco-Associated Oral Mucosal Lesions Érica Dorigatti de Ávila1, Rafael Scaf de Molon2, Melaine de Almeida Lawall1, Renata Bianco Consolaro1, Alberto Consolaro1 Variant Branching Pattern of the Right Internal Iliac Vessels in A Male: A Case Report Satheesha Nayak Badagabettu, Naveen Kumar, Surekha Devadasa Shetty, Srinivasa Rao Sirasanagandla 1Bauru Dental School Abstract University of São Paulo Department of AnatomyBauru–SP, Brazil AbstractObjective: To evaluate the changes in the number of Langerhans Cells (LC) observed in the epitheliumMelaka ofManipal Medical College 2Araraquara Dental School smokeless tobacco (SLT-induced) lesions. (Manipal Campus) Internal iliac vessels show frequent variations in their branching pattern. We saw variations in the São Paulo State University Methods: Microscopic sections from biopsies carried out in the buccal mucosa of twenty patients, whoManipal were University branching pattern of right internal iliac vessels in a male cadaver. The internal iliac artery did not divide Manipal, Karnataka,Araraquara-SP, India Brazil intochronic anterior users and of posteriorsmokeless divisions. tobacco There (SLT), were were three utilized. common For thetrunks: control one group,for iliolumbar twenty andnon-SLT lateral users of SLT Received: Aug 09,Received: 2012 February 05, 2012 sacralwith normalarteries, mucosa another forwere inferior selected. gluteal The and sections internal werepudendal studied arteries, with routineand the thirdcoloring one forand superior were immunostained Accepted: Oct 09,Accepted: 2012 February 29, 2012 vesicalfor S-100, and CD1a,obturator Ki-67 arteries. and p63.The Thesesuperior data gluteal were and statistically middle rectal analyzed arteries by thearose Student’s directly t-testfrom tothe investigate Arch Clin the Exp SurgArch 2014;3:197-200 Clin Exp Surg 2012;X: X-X DOI:10.5455/aces.20121009120145 maindifferences trunk of in the the internal expression iliac artery. -

Surgical Anatomy of the Common Iliac Veins During Para-Aortic and Pelvic Lymphadenectomy for Gynecologic Cancer
Original Article J Gynecol Oncol Vol. 25, No. 1:64-69 http://dx.doi.org/10.3802/jgo.2014.25.1.64 pISSN 2005-0380·eISSN 2005-0399 Surgical anatomy of the common iliac veins during para-aortic and pelvic lymphadenectomy for gynecologic cancer Kazuyoshi Kato, Shinichi Tate, Kyoko Nishikimi, Makio Shozu Department of Gynecology, Chiba University School of Medicine, Chiba, Japan See accompanying editorial by Lee on page 1. Objective: Compression of the left common iliac vein between the right common iliac artery and the vertebrae is known to be associated with the occurrence of left iliofemoral deep vein thrombosis (DVT). In this study, we described the variability in vascular anatomy of the common iliac veins and evaluated the relationship between the degree of iliac vein compression and the presence of DVT using the data from surgeries for gynecologic cancer. Methods: The anatomical variations and the degrees of iliac vein compression were determined in 119 patients who underwent systematic para-aortic and pelvic lymphadenectomy during surgery for primary gynecologic cancer. Their medical records were reviewed with respect to patient-, disease-, and surgery-related data. Results: The degrees of common iliac vein compression were classified into three grades: grade A (n=28, 23.5%), with a calculated percentage of 0%-25% compression; grade B (n=47, 39.5%), with a calculated percentage of 26%-50% compression; and grade C (n=44, 37%), with a calculated percentage of more than 50% compression. Seven patients (5.9%) had common iliac veins with anomalous anatomies; three were divided into small caliber vessels, two with a flattened structure, and two had double inferior vena cavae. -

Pelvic Venous Disorders
PELVIC VENOUS DISORDERS Anatomy and Pathophysiology Two Abdomino-Pelvic Compression Syndromes DIAGNOSIS of ABDOMINOO-PELVICP z Nutcracker Syndrome 9 Compression of the left renal vein COMPRESSIONCO SS O SYNDROMES S O with venous congestion of the left (with Emphasis on Duplex Ultrasound) kidney and left ovarian vein reflux R. Eugene Zierler, M.D. z May-Thurner Syndrome 9 Compression of the left common iliac vein by the right common The DD.. EE.. StrandnessStrandness,, JrJr.. Vascular Laboratory iliac artery with left lower University of Washington Medical Center extremity venous stasis and left DivisionDivision of Vascular Surgery internal iliac vein reflux University of Washington, School of Medicine ABDOMINO-PELVIC COMPRESSION Nutcracker Syndrome Left Renal Vein Entrapment z Grant 1937: Anatomical observation “…the left renal vein, as it lies between the aorta and superior mesenteric artery, resembles a nut between the jaws of a nutcracker.” X z El-Sadr 1950: Described first patient with the clinical syndrome X z De Shepper 1972: Named the disorder “Nutcracker Syndrome” Copy Here z Nutcracker Phenomenon z Nutcracker Syndrome 9 Anatomic finding only 9 Hematuria, proteinuria 9 Compression of left renal 9 Flank pain vein - medial narrowing 9 Pelvic pain/congestion with lateral (hilar) dilation 9 Varicocele ABDOMINO-PELVIC COMPRESSION ABDOMINO-PELVIC COMPRESSION Nutcracker Syndrome - Diagnosis Nutcracker Syndrome z Anterior Nutcracker z Posterior Nutcracker z Evaluate the left renal vein for aorto-mesenteric compression 9 Compression between -

Pelvic Venous Reflux Diseases
Open Access Journal of Family Medicine Review Article Pelvic Venous Reflux Diseases Arbid EJ* and Antezana JN Anatomic Considerations South Charlotte General and Vascular Surgery, 10512 Park Road Suite111, Charlotte, USA Each ovary is drained by a plexus forming one major vein *Corresponding author: Elias J. Arbid, South measuring normally 5mm in size. The left ovarian plexus drains into Charlotte General and Vascular Surgery, 10512 Park Road left ovarian vein, which empties into left renal vein; the right ovarian Suite111, Charlotte, NC 28120, USA plexus drains into the right ovarian vein, which drains into the Received: November 19, 2019; Accepted: January 07, anterolateral wall of the inferior vena cava (IVC) just below the right 2020; Published: January 14, 2020 renal vein. An interconnecting plexus of veins drains the ovaries, uterus, vagina, bladder, and rectum (Figure 1). Introduction The lower uterus and vagina drain into the uterine veins and Varicose veins and chronic venous insufficiency are common then into branches of the internal iliac veins; the fundus of the uterus disorders of the venous system in the lower extremities that have drains to either the uterine or the ovarian plexus (utero-ovarian and long been regarded as not worthy of treatment, because procedures salpingo ovarian veins) within the broad ligament. Vulvoperineal to remove them were once perceived as worse than the condition veins drain into the internal pudendal vein, then into the inferior itself. All too frequently, patients are forced to learn to live with them, gluteal vein, then the external pudendal vein, then into the saphenous or find "creative" ways to hide their legs. -
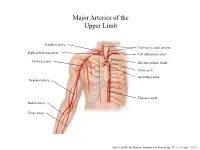
Major Arteries of the Upper Limb
Major Arteries of the Upper Limb Vertebral artery Common carotid arteries Right subclavian artery Left subclavian artery Axillary artery Brachiocephalic trunk Aortic arch Ascending aorta Brachial artery Thoracic aorta Radial artery Ulnar artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.23 Major Arteries of the Abdominal Region Renal artery Celiac trunk Abdominal aorta Superior mesenteric artery Gonadal artery Inferior mesenteric artery Common iliac artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.24 Common iliac artery Internal iliac artery Major Arteries of the External iliac artery Lower Limb Femoral artery Popliteal artery Anterior tibial artery Fibular artery Posterior tibial artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.25 Major Veins of the Upper Limb Internal jugular vein (left) Subclavian vein (right) External jugular vein (left) Axillary vein Brachiocephalic veins Cephalic vein Superior vena cava Brachial vein Basilic vein Median cubital vein Inferior vena cava Radial vein Ulnar vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.28 Major Veins of the Abdominal Cavity – Part 1 Hepatic veins Inferior vena cava Renal vein (left) Gonadal vein (left) Gonadal vein (right) Common iliac vein (left) Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.29 Major Veins of the Abdominal Cavity – Part 2 (Hepatic portal circulation) Hepatic portal vein Splenic vein Inferior mesenteric vein Superior mesenteric vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.29 Common iliac vein (left) Internal iliac vein Major Veins of the External iliac vein Lower Limb Great saphenous vein Femoral vein Popliteal vein Fibular vein Small saphenous vein Anterior tibial Posterior tibial vein vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.30 . -

A Ureteral Mass Presenting As Deep Vein Thrombosis: a Very Rare Presentation
Urology & Nephrology Open Access Journal Case Report Open Access A ureteral mass presenting as deep vein thrombosis: a very rare presentation Abstract Volume 6 Issue 3 - 2018 Venous Thromboembolism is a dreaded condition. Many conditions and systemic Dushiant Sharma, Umesh Sharma, Sumit diseases are known to predispose to its occurrence including malignancy, pancreatitis, burns, clotting disorders and direct compression. All of these have been known to affect Gehlawat Department of Urology, RML Hospital & PGIMER, Delhi, India one or more factors in Virchows Triad proposed roughly a century ago.1 It consists of Hypercoagulability, Vascular Endothelial Dysfunction, Stasis. Stasis, one of the factors in Correspondence: Dushiant Sharma, Department of Urology, Virchows triad has been studied to a very small extent and it is this feature that is mostly Dr RML Hospital & PGIMER, Baba Khadak Singh Marg, New responsible for the development of Deep Vein Thrombosis (DVT) due to direct compression. Delhi, 110001, Tel 011-2340-4323, Email [email protected] Iliofemoral thrombosis in malignancy patients can be caused due to vein compression by pelvic malignancy and usually presents as unilateral lower limb swelling. In patients with Received: November 15, 2018 | Published: June 22, 2018 sudden onset of unilateral lower limb swelling without any perceived or diagnosed medical condition, detailed evaluation may lead to early identification, appropriate management and possibly cure. We present a case of acute unilateral ilio-femoral DVT caused by external compression by a mid-ureteric mass. Keywords: deep vein thrombosis, ileofemoral thrombosis, ureteric malignancy, upper tract urothelial cancers Abbreviations: DVT, Deep Vein Thrombosis; VTE, Venous Nephrouretectomy with bladder cuff excision was planned. -

A Case of the Bilateral Superior Venae Cavae with Some Other Anomalous Veins
Okaiimas Fol. anat. jap., 48: 413-426, 1972 A Case of the Bilateral Superior Venae Cavae With Some Other Anomalous Veins By Yasumichi Fujimoto, Hitoshi Okuda and Mihoko Yamamoto Department of Anatomy, Osaka Dental University, Osaka (Director : Prof. Y. Ohta) With 8 Figures in 2 Plates and 2 Tables -Received for Publication, July 24, 1971- A case of the so-called bilateral superior venae cavae after the persistence of the left superior vena cava has appeared relatively frequent. The present authors would like to make a report on such a persistence of the left superior vena cava, which was found in a routine dissection cadaver of their school. This case is accompanied by other anomalies on the venous system ; a complete pair of the azygos veins, the double subclavian veins of the right side and the ring-formation in the left external iliac vein. Findings Cadaver : Mediiim nourished male (Japanese), about 157 cm in stature. No other anomaly in the heart as well as in the great arteries is recognized. The extracted heart is about 350 gm in weight and about 380 ml in volume. A. Bilateral superior venae cavae 1) Right superior vena cava (figs. 1, 2, 4) It measures about 23 mm in width at origin, about 25 mm at the pericardiac end, and about 31 mm at the opening to the right atrium ; about 55 mm in length up to the pericardium and about 80 mm to the opening. The vein is formed in the usual way by the union of the right This report was announced at the forty-sixth meeting of Kinki-district of the Japanese Association of Anatomists, February, 1971,Kyoto. -

Sources of Venous Blood Supply of Kidneys in Chicken of Haysex White Breed
M. V. Pervenetskaya et al /J. Pharm. Sci. & Res. Vol. 10(10), 2018, 2639-2641 Sources of Venous Blood Supply of Kidneys in Chicken of Haysex White Breed M. V. Pervenetskaya, L. V. Fomenko, G. A. Honin Omsk State Agrarian University named after P.A. Stolypin, 644008, Omsk, Russian Federation, Institutskaya Square, 1 Abstract: As a result of our studies, it has been found that blood from pelvic extremities in chicken flows along the right and left external iliac veins into the pelvic cavity. The caudal portal vein of the kidney branches from it in the caudal direction - from cranial part of kidneys. The caudal portal vein collects blood from middle and caudal parts of the kidney. The caudal portal vein, slightly bending in the caudomedial direction, joins the caudal portal vein from the opposite side. Coccygeal mesenteric vein branches from junctions of these veins. The right and left renal veins when joining together with an acute angle flow into the common iliac vein, which in turn enters caudal vena cava. Intraorgan veins of the kidney are divided into interlobular, perilobular, intralobular veins and capillaries. Venous blood from kidneys flows by three different ways. The first way is carried out by the hepatic veins from the midst and caudal parts of kidney. Firstly, these veins flow into the common iliac vein, and then into the caudal part of vena cava. The second way of outflow is carried out through the coccygeal mesenteric vein in the liver. The third way is carried out along the right and left cranial anterior portal veins from the anterior part of the kidney into the vertebral venous canal.