Thesis by Michelle-Nicole Havlik
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotechnologies from Marine Bivalves
Nutrient Extraction Through Bivalves Petersen, Jens Kjerulf; Holmer, Marianne; Termansen, Mette; Hasler, Berit Published in: Goods and Services of Marine Bivalves DOI: 10.1007/978-3-319-96776-9_10 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Petersen, J. K., Holmer, M., Termansen, M., & Hasler, B. (2019). Nutrient Extraction Through Bivalves. In A. C. Smaal, J. G. Ferreira, J. Grant, J. K. Petersen, & Ø. Strand (Eds.), Goods and Services of Marine Bivalves (pp. 179-208). Springer. https://doi.org/10.1007/978-3-319-96776-9_10 Download date: 05. okt.. 2021 Aad C. Smaal · Joao G. Ferreira · Jon Grant Jens K. Petersen · Øivind Strand Editors Goods and Services of Marine Bivalves Goods and Services of Marine Bivalves Just the pearl II, by Frank van Driel, fine art photography (www.frankvandriel.com), with painted oyster shells of www.zeeuwsblauw.nl Aad C. Smaal • Joao G. Ferreira • Jon Grant Jens K. Petersen • Øivind Strand Editors Goods and Services of Marine Bivalves Editors Aad C. Smaal Joao G. Ferreira Wageningen Marine Research and Universidade Nova de Lisboa Aquaculture and Fisheries group Monte de Caparica, Portugal Wageningen University and Research Yerseke, The Netherlands Jens K. Petersen Technical University of Denmark Jon Grant Nykøbing Mors, Denmark Department of Oceanography Dalhousie University Halifax, Nova Scotia, Canada Øivind Strand Institute of Marine Research Bergen, Norway ISBN 978-3-319-96775-2 ISBN 978-3-319-96776-9 (eBook) https://doi.org/10.1007/978-3-319-96776-9 Library of Congress Control Number: 2018951896 © The Editor(s) (if applicable) and The Author(s) 2019 , corrected publication 2019. -
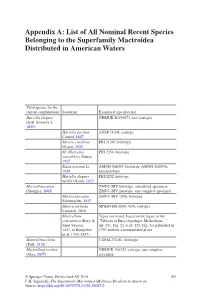
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Supplement – December 2017 – Survey of the Literature on Recent
A Malacological Journal ISSN 1565-1916 No. 36 - SUPPLEMENT DECEMBER 2017 2 SURVEY OF THE LITERATURE ON RECENT SHELLS FROM THE RED SEA (third enlarged and revised edition) L.J. van Gemert* Summary This literature survey lists approximately 3,050 references. Shells are being considered here as the shell bearing molluscs of the Gastropoda, Bivalvia and Scaphopoda. The area does not only comprise the Red Sea, but also the Gulf of Aden, Somalia and the Suez Canal, including the Lessepsian species in the Mediterranean Sea. Literature on fossils shells, particularly those from the Holocene, Pleistocene and Pliocene, is listed too. Introduction My interest in recent shells from the Red Sea dates from about 1996. Since then, I have been, now and then, trying to obtain information on this subject. Some years ago I decide to stop gathering data in a haphazard way and to do it more properly. This resulted in a first survey of approximately 1,420 and a second one of 2,025 references (van Gemert, 2010 & 2011). Since then, this survey has again been enlarged and revised and a number of errors have been corrected. It contains now approximately 3,050 references. Scope In principle every publication in which molluscs are reported to live or have lived in the Red Sea should be listed in the survey. This means that besides primary literature, i.e. articles in which researchers are reporting their finds for the first time, secondary and tertiary literature, i.e. reviews, monographs, books, etc are to be included too. These publications were written not only by a wide range of authors ranging from amateur shell collectors to professional malacologists but also people interested in the field of archaeology, geology, etc. -

Apo-Nid110846.Pdf
http://researchspace.auckland.ac.nz ResearchSpace@Auckland Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: • Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. • Authors control the copyright of their thesis. You will recognise the author's right to be identified as the author of this thesis, and due acknowledgement will be made to the author where appropriate. • You will obtain the author's permission before publishing any material from their thesis. To request permissions please use the Feedback form on our webpage. http://researchspace.auckland.ac.nz/feedback General copyright and disclaimer In addition to the above conditions, authors give their consent for the digital copy of their work to be used subject to the conditions specified on the Library Thesis Consent Form and Deposit Licence. Enhancing larval survival and re-introduction techniques for Tridacnidae (giant clams): Reversing a path toward extinction Charles George Waters Institute of Marine Science University of Auckland A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in Marine Science, The University of Auckland, 2014 Abstract Mariculture interest in the Tridacnidae family of giant clams has increased in response to economic and nutritional opportunities, but also in response to increasing reports of local extinctions as a result of over-harvesting, pollution, and habitat degradation. -

Giant Clams in a Changing Ocean
M 6494 GIANT CLAMS IN A CHANGING OCEAN: Effects of Ocean Warming and Acidification on Tridacna maxima, a Solar-powered Bivalve CATARINA DA CONCEIÇÃO PEREIRA SANTOS DISSERTAÇÃO DE MESTRADO EM CIÊNCIAS DO MAR – RECURSOS MARINHOS SUBMETIDA AO INSTITUTO DE CIÊNCIAS BIOMÉDICAS ABEL SALAZAR DA UNIVERSIDADE DO PORTO 1 2 CATARINA DA CONCEIÇÃO PEREIRA SANTOS GIANT CLAMS IN A CHANGING OCEAN: Effects of Ocean Warming and Acidification on Tridacna maxima, a Solar-powered Bivalve Dissertação de Candidatura ao grau de Mestre em Ciências do Mar – Recursos Marinhos submetida ao Instituto de Ciências Biomédicas de Abel Sala- zar da Universidade do Porto. Orientadores: Doutor Rui Rosa Investigador Principal MARE | Centro de Ciências do Mar e do Ambiente Doutor Jorge Machado Professor Associado ICBAS | Instituto de Ciências Biomédicas Abel Sa- lazar da Universidade do Porto 3 4 ACKNOWLEDGEMENTS I would like to express my gratitude towards the people who made this work possible: To Doctor Rui Rosa, my supervisor at Laboratório Marítimo da Guia, for providing me with the opportunity, tools and guidance that made it all possible. With his open-mind and al- ways-curious personality I earned an amazing example of scientist to look for. To Doctor Jorge Machado, my supervisor from ICBAS, who supported me through all this process with all his enthusiasm for the theme in question and the techniques I was able to learn and apply throughout this journey. To Gisela Dionísio, for coming up with this idea and being there at every step, as the wise and comprehensive voice of experience. To Ricardo Cyrne, the handy-man of the team. -
SURVIVORSHIP, GROWTH and PIGMENTATION RESPONSES of the MARINE ORNAMENTAL INVERTEBRATE Tridacna Maxima to VARIED IRRADIANCE LEVELS in TWO DIFFERENT CULTURE SYSTEMS
SURVIVORSHIP, GROWTH AND PIGMENTATION RESPONSES OF THE MARINE ORNAMENTAL INVERTEBRATE Tridacna maxima TO VARIED IRRADIANCE LEVELS IN TWO DIFFERENT CULTURE SYSTEMS By MICAH ALO A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Micah Alo This document is dedicated to the friends and supporters of the marine ornamental hobby. ACKNOWLEDGMENTS This thesis would not have been possible without the help of many individuals. First, I thank all my committee members Dr. Charles Cichra, Dr. Shirley Baker, Dr. Daryl Parkyn and Dr. John Baldwin, for their encouragement and much needed enlightenment. This project was made possible with funding provided by the USDA CS-REES Special Research Grants and funding from the University of Florida’s College of Agricultural and Life Sciences and Department of Fisheries and Aquatic Sciences. I owe a lot of thanks to the staff and students of the University of Florida’s Tropical Aquaculture Lab. I thank Craig Watson for his belief in the advancement of aquaculture and giving this project its direction. Scott Graves, Robert Leonard, and Tina Crosby all deserve credit for building the greenhouse and systems. Fellow graduate student and tinkerer Jon Kao was instrumental for aiding in tank design and construction, ammonia readings; and most other problems that I encountered during my study. Also, I need to thank Dr. Jeff Hill for his statistical analysis and Dr. Roy Yanong for clam disease diagnostics and all the mentoring they bestowed upon me. -

(Tridacnidae) and CITES Capacity Building SPC Aquaculture Technical Papers
SPC Aquaculture Technical Papers ISSN: 0377-452X Proceedings of the Regional Workshop on the Management of Sustainable Fisheries for Giant Clams (Tridacnidae) and CITES Capacity Building SPC Aquaculture Technical Papers Proceedings of the Regional Workshop on the Management of Sustainable Fisheries for Giant Clams (Tridacnidae) and CITES Capacity Building 4–7 August 2009 Nadi, Fiji Jeff Kinch and Antoine Teitelbaum Secretariat of the Pacific Community Noumea, New Caledonia 2010 © Copyright Secretariat of the Pacific Community (SPC) 2010 All rights for commercial / for profit reproduction or translation, in any form, reserved. SPC authorises the partial reproduction or translation of this material for scientific, educational or research purposes, provided SPC and the source document are properly acknowledged. Permission to reproduce the document and/or translate in whole, in any form, whether for commercial / for profit or non-profit purposes, must be requested in writing. Original SPC artwork may not be altered or separately published without permission. Original text: English Secretariat of the Pacific Community Cataloguing-in-publication data Kinch, Jeff Proceedings of the regional workshop on the management of sustainable fisheries for giant clams (Tridacnidae) and CITES capacity building (4–7 August 2009, Nadi, Fiji) / by Jeff Kinch and Antoine Teitelbaum (Report of meeting (Technical) / Secretariat of the Pacific Community) ISSN: 0377-452X Giant clams — Oceania — Congresses. Shellfish trade — Management — Oceania — Congresses. Clam -

Review of Species Selected on the Basis of an Overview of Long-Standing Positive Opinions Part Ii
UNEP-WCMC technical report REVIEW OF SPECIES SELECTED ON THE BASIS OF AN OVERVIEW OF LONG-STANDING POSITIVE OPINIONS PART II (Version edited for public release) Review of species selected on the basis of an overview of long- standing positive opinions. Part II Prepared for The European Commission, Directorate General Environment, Directorate F - Global Sustainable Development, Unit F3 - Multilateral Environmental Cooperation, Brussels, Belgium. Published February 2020 Copyright European Commission 2020 Citation UNEP-WCMC. 2020. Review of species selected on the basis of an overview of long-standing positive opinions. Part II. UNEP-WCMC, Cambridge. Cover Photo Caiman crocodilus yacare, Pantanal, Brazil. andreanita / Adobe Stock The UN Environment Programme World Conservation Monitoring Centre (UNEP-WCMC) is a global Centre of excellence on biodiversity. The Centre operates as a collaboration between the UN Environment Programme and the UK-registered charity WCMC. Together we are confronting the global crisis facing nature. This publication may be reproduced for educational or non-profit purposes without special permission, provided acknowledgement to the source is made. Reuse of any figures is subject to permission from the original rights holders. No use of this publication may be made for resale or any other commercial purpose without permission in writing from the UN Environment Programme. Applications for permission, with a statement of purpose and extent of reproduction, should be sent to the Director, UNEP-WCMC, 219 Huntingdon Road, -

Tridacna Maxima Röding, 1798
AC22 Doc. 10.2 Annex 8f Tridacna maxima Röding, 1798 FAMILY: Tridacnidae COMMON NAMES: Maxima Clam; Small Giant Clam (English) GLOBAL CONSERVATION STATUS: Listed as Lower Risk/Conservation Dependent in the 2004 IUCN Red List of Threatened Species (IUCN, 2004). SIGNIFICANT TRADE REVIEW FOR: Australia, Comoros, Egypt, Fiji, French Polynesia (France), India, Japan, Kenya, Kiribati, Madagascar, Malaysia, Marshall Islands, Mauritius, Mayotte (France), Micronesia, Mozambique, Myanmar, New Caledonia (France), Niue, Palau, Papua New Guinea, Pitcairn Islands (United Kingdom), Réunion (France), Saudi Arabia, Somalia, Sri Lanka, Tonga, United Republic of Tanzania, Vanuatu, Viet Nam, Wallis and Futuna Islands (France) Range States selected for review Range State Exports* Urgent, possible Comments (1994-2003) or least concern Australia 5,386 live Least concern Trade almost entirely in captive-bred specimens. captive Comoros None Least concern No trade reported Egypt Minimal trade Least concern Minimal trade since 1999 since 1999 Federated States 18,679 live Possible concern Population declines in heavily fished areas; high exports of Micronesia wild from wild sources mainly for aquarium trade; domestic use levels unknown; confusion over commercial trade regulations/ban. Fiji ca 28,000 live Possible concern Significant trade in will specimens (though none recorded wild since 2002); domestic use levels unknown; status unknown; no population monitoring or basis for non- detriment findings available. French Polynesia Low trade Least concern Minimal trade -

An Analysis of the Import of Live Animals from Indonesia by the Netherlands
TRAFFIC GOING DUTCH REPORT An analysis of the import of live animals from Indonesia by the Netherlands NOVEMBER 2016 Jordi Janssen and Lisa J. Blanken TRAFFIC Report: Going Dutch: An analysis of the import of live animals from Indonesia by the Netherlands 1 TRAFFIC REPORT TRAFFIC, the wild life trade monitoring net work, is the leading non-governmental organization working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. TRAFFIC is a strategic alliance of WWF and IUCN. Reprod uction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations con cern ing the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views of the authors expressed in this publication are those of the writers and do not necessarily reflect those of TRAFFIC, WWF or IUCN. Published by TRAFFIC. Southeast Asia Regional Office Unit 3-2, 1st Floor, Jalan SS23/11 Taman SEA, 47400 Petaling Jaya Selangor, Malaysia Telephone : (603) 7880 3940 Fax : (603) 7882 0171 Copyright of material published in this report is vested in TRAFFIC. © TRAFFIC 2016. ISBN no: 978-983-3393-67-1 UK Registered Charity No. 1076722. Suggested citation: Janssen, J. and Blanken, L.J. (2016). Going Dutch: An analysis of the import of live animals from Indonesia by the Netherlands. -

Review of Species Subject to Long-Standing Positive Opinions: Species Other Than Corals and Butterflies from Asia and Oceania
Review of species subject to long-standing positive opinions: species other than corals and butterflies from Asia and Oceania (Version edited for public release) Prepared for the European Commission Directorate General E - Environment ENV.E.2. – Development and Environment by the United Nations Environment Programme World Conservation Monitoring Centre August, 2012 UNEP World Conservation Monitoring Centre PREPARED FOR 219 Huntingdon Road The European Commission, Brussels, Belgium Cambridge CB3 0DL United Kingdom Tel: +44 (0) 1223 277314 DISCLAIMER Fax: +44 (0) 1223 277136 The contents of this report do not necessarily reflect Email: [email protected] the views or policies of UNEP or contributory Website: www.unep-wcmc.org organisations. The designations employed and the presentations do not imply the expressions of any The United Nations Environment Programme opinion whatsoever on the part of UNEP, the World Conservation Monitoring Centre (UNEP- European Commission or contributory WCMC) is the specialist biodiversity assessment organisations concerning the legal status of any centre of the United Nations Environment country, territory, city or area or its authority, or Programme (UNEP), the world’s foremost concerning the delimitation of its frontiers or intergovernmental environmental organisation. boundaries. The Centre has been in operation for over 30 years, combining scientific research with practical policy advice. The Centre's mission is to evaluate and © Copyright: 2012, European Commission highlight the many values of biodiversity and put authoritative biodiversity knowledge at the centre of decision-making. Through the analysis and synthesis of global biodiversity knowledge the Centre provides authoritative, strategic and timely information for conventions, countries and organisations to use in the development and implementation of their policies and decisions. -

The Philippine Shell Industry with Special Focus on Mactan, Cebu
THE PHILIPPINE SHELL INDUSTRY WITH SPECIAL FOCUS ON MACTAN, CEBU ADONIS S. FLOREN Coastal Resource Management Project of the Department of Environment and Natural Resources Supported by the United States Agency for International Development 2003 1 Table of Contents List of Acronyms List of Tables and Figures Acknowledgements Preface Introduction History of the Shell Trade in the Philippines Profile of the Industry The Development of Mactan Shell Industry Current Reality Classification of Products Collection Practices Methods of Collection The Process of Cleaning Philippine Collection Sites Export Production Volume of Trade Current Number of Shell Establishments Perceptions and Problems of the Industry Trends in the Supply and Demand of Shells Problems of the Industry Legal and Conservation Measures History of Legislation Conclusion Recommendations References Appendix 2 List of Acronyms and Abbreviations BAS Bureau of Agricultural Statistics BFAR Bureau of Fisheries and Aquatic Resources CITES Convention on International Trade in Endangered Species of Wild Flora and Fauna CPO Cebu Provincial Office CRMP Coastal Resource Management Project DA Department of Agriculture DENR Department of Environment and Natural Resources DILG Department of the Interior and Local Government DTI Department of Trade and Industry FA Fashion Accessories FAO Fisheries Administrative Order FARMC Fisheries and Aquatic Resource Management Council FAME Fashion Accessories Manufacturers and Exporters ICLARM International Center for Living Aquatic Resources Management LGU Local Government Unit kg Kilogram MOP Mother-of-Pearl NACIDA National Cottage Industry Development Authority NGO Non-government Organization PhP Philippine Peso RA Republic Act SUML Silliman University Marine Laboratory t ton TESDA Technical Education and Skills Development Authority USC University of San Carlos UP-MSI University of the Philippines-Marine Science Institute USAID United States Agency for International Development $ US dollar Exchange rate: P50 = US$1.