Sero-Prevalence and Risk Factors of Foot And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ADDENDUM – TENDER NO BEC EAC 009/12-13 the Provision of Security Printing and Packaging Services for the Primary School Leavin
ADDENDUM – TENDER NO BEC EAC 009/12-13 The Provision of Security Printing and Packaging Services for The Primary School Leaving Examinations (PSLE), Junior Certificate Examination (JCE) for 2013, 2014 and 2015 Examinations for Botswana Examinations Council Please find an addendum that contains additional information of number of centres and regional offices for the above stated tender. PRIMARY SCHOOL CENTRES NAME OF INSPECTORAL CENTRE SCHOOL PLACE DISTRICT AREA ADDRESS TEL.NO CEL NO. NO. Jamataka P O Box 1980 Primary School Tutume Central Tutume east Francistown 73462387 PS0839 Legotlhwana P O Box 104 Primary School Sehithwa Ngami Ngamiland Sehithwa 6860241 71520531 PS1921 Thuto Primary Molepolole P O Box 1296 School Mogoditshane Kweneng region Mogoditshane 3906407 71792509 PS1443 Foley Itireleng Tonota region P O Box 79 Primary School Tonota Central Tonota 2484226 PS0237 Kubung Primary P/Bag B03 School Maun Ngami Maun region Boseja, Maun 6861487 73836358 PS1830 Patayamatebele P O Box Primary School Patayamatebele North North East East 150126 71730730 East Tonota 71207130 PS1644 Samochima P O Box 366 Primary School Samochima Ngami Okavango Shakawe 73850820 PS2027 St Anthony’s Palapye North P O Box 005 Primary School Palapye Central Palapye 71657665 PS0747 P O Box 176 Nokaneng Nokaneng Ngami Okavango Gumare 6874700 PS2028 OSET P O Box 176 Qangwa OSET Qangwa Ngami Okavango Gumare 6874700 PS2029 Centre Name Address Place Phone 0101 Bobonong Primary School P.O. Box 48 BOBONONG 2619207 0103 Borotsi Primary School P.O. Box 136 BOBONONG 819208 0107 Gobojango Primary School Private Bag 8 BOBONONG 2645436 0108 Lentswe-Le-Moriti Primary School Private Bag 0019 BOBONONG 0110 Mabolwe Primary School P.O. -
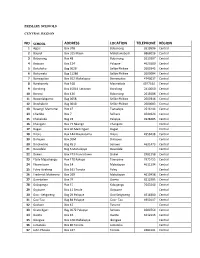
Public Primary Schools
PRIMARY SCHOOLS CENTRAL REGION NO SCHOOL ADDRESS LOCATION TELE PHONE REGION 1 Agosi Box 378 Bobonong 2619596 Central 2 Baipidi Box 315 Maun Makalamabedi 6868016 Central 3 Bobonong Box 48 Bobonong 2619207 Central 4 Boipuso Box 124 Palapye 4620280 Central 5 Boitshoko Bag 002B Selibe Phikwe 2600345 Central 6 Boitumelo Bag 11286 Selibe Phikwe 2600004 Central 7 Bonwapitse Box 912 Mahalapye Bonwapitse 4740037 Central 8 Borakanelo Box 168 Maunatlala 4917344 Central 9 Borolong Box 10014 Tatitown Borolong 2410060 Central 10 Borotsi Box 136 Bobonong 2619208 Central 11 Boswelakgomo Bag 0058 Selibe Phikwe 2600346 Central 12 Botshabelo Bag 001B Selibe Phikwe 2600003 Central 13 Busang I Memorial Box 47 Tsetsebye 2616144 Central 14 Chadibe Box 7 Sefhare 4640224 Central 15 Chakaloba Bag 23 Palapye 4928405 Central 16 Changate Box 77 Nkange Changate Central 17 Dagwi Box 30 Maitengwe Dagwi Central 18 Diloro Box 144 Maokatumo Diloro 4958438 Central 19 Dimajwe Box 30M Dimajwe Central 20 Dinokwane Bag RS 3 Serowe 4631473 Central 21 Dovedale Bag 5 Mahalapye Dovedale Central 22 Dukwi Box 473 Francistown Dukwi 2981258 Central 23 Etsile Majashango Box 170 Rakops Tsienyane 2975155 Central 24 Flowertown Box 14 Mahalapye 4611234 Central 25 Foley Itireleng Box 161 Tonota Foley Central 26 Frederick Maherero Box 269 Mahalapye 4610438 Central 27 Gasebalwe Box 79 Gweta 6212385 Central 28 Gobojango Box 15 Kobojango 2645346 Central 29 Gojwane Box 11 Serule Gojwane Central 30 Goo - Sekgweng Bag 29 Palapye Goo-Sekgweng 4918380 Central 31 Goo-Tau Bag 84 Palapye Goo - Tau 4950117 -

Daily Hansard 05 March 2020 Budget
DAILY YOUR VOICE IN PARLIAMENT THE FIRSTTHE SECOND MEETING MEETING OF THE OF THE FIFTH FIRST SESSION SESSION OF THEOF ELEVENTH THE TWELFTH PARLIAMENT PARLIAMENT WEDNESDAYTUESDAYTHURSDAY 13 0705 NOVEMBER NOVEMBERMARCH 2020 2018 2018 ENGLISH VERSION HANSARDHANSARD NO. 192196 DISCLAIMER Unofficial Hansard This transcript of Parliamentary proceedings is an unofficial version of the Hansard and may contain inaccuracies. It is hereby published for general purposes only. The final edited version of the Hansard will be published when available and can be obtained from the Assistant Clerk (Editorial). THE NATIONAL ASSEMBLY SPEAKER The Hon. Phandu T. C. Skelemani PH, MP. DEPUTY SPEAKER The Hon. Mabuse M. Pule, MP. (Mochudi East) Clerk of the National Assembly - Ms B. N. Dithapo Deputy Clerk of the National Assembly - Mr L. T. Gaolaolwe Learned Parliamentary Counsel - Ms M. Mokgosi Assistant Clerk (E) - Mr R. Josiah CABINET His Excellency Dr M. E. K. Masisi, MP. - President His Honour S. Tsogwane, MP. (Boteti West) - Vice President Minister for Presidential Affairs, Governance and Public Hon. K. N. S. Morwaeng, MP. (Molepolole South) - Administration Hon. K. T. Mmusi, MP. (Gabane-Mmankgodi) - Minister of Defence, Justice and Security Hon. Dr U. Dow, MP. (Specially Elected) - Minister of International Affairs and Cooperation Hon. E. M. Molale, MP. (Goodhope-Mabule ) - Minister of Local Government and Rural Development Hon. Dr E. G. Dikoloti MP. (Mmathethe-Molapowabojang) - Minister of Agricultural Development and Food Security Minister of Environment, Natural Resources Conservation Hon. P. K. Kereng, MP. (Specially Elected) - and Tourism Hon. Dr L. Kwape, MP. (Kanye South) - Minister of Health and Wellness Hon. T.M. Segokgo, MP. -

Daily Hansard 16 March 2021
DAILY YOUR VOICE IN PARLIAMENT THE SECONDSECOND MEETING MEETING OF THEOF SECONDTHE FIFTH SESSION SESSION OF THEOF THEELEVEN TWELFTHTH PARLIAMENT PARLIAMENT TUESDAY 16 MARCH 2021 MIXEDENGLISH VERSION VERSION HANSARDHANSARD NO. 193201 DISCLAIMER Unocial Hansard This transcript of Parliamentary proceedings is an unocial version of the Hansard and may contain inaccuracies. It is hereby published for general purposes only. The nal edited version of the Hansard will be published when available and can be obtained from the Assistant Clerk (Editorial). THE NATIONAL ASSEMBLY SPEAKER The Hon. Phandu T. C. Skelemani PH, MP. DEPUTY SPEAKER The Hon. Mabuse M. Pule, MP. (Mochudi East) Clerk of the National Assembly - Ms B. N. Dithapo Deputy Clerk of the National Assembly - Mr L. T. Gaolaolwe Learned Parliamentary Counsel - Ms M. Mokgosi Assistant Clerk (E) - Mr R. Josiah CABINET His Excellency Dr M. E. K. Masisi, MP. - President His Honour S. Tsogwane, MP. (Boteti West) - Vice President Minister for Presidential Affairs, Governance and Public Hon. K. N. S. Morwaeng, MP. (Molepolole South) - Administration Hon. K. T. Mmusi, MP. (Gabane-Mmankgodi) - Minister of Defence, Justice and Security Hon. Dr L. Kwape, MP. (Kanye South) - Minister of International Affairs and Cooperation Hon. E. M. Molale, MP. (Goodhope-Mabule ) - Minister of Local Government and Rural Development Hon. K. S. Gare, MP. (Moshupa-Manyana) - Minister of Agricultural Development and Food Security Minister of Environment, Natural Resources Conservation Hon. P. K. Kereng, MP. (Specially Elected) - and Tourism Hon. Dr E. G. Dikoloti MP. (Mmathethe-Molapowabojang) - Minister of Health and Wellness Hon. T.M. Segokgo, MP. (Tlokweng) - Minister of Transport and Communications Hon. -

Engineering Design and Tender Documentation for Francistown Water Supply Infrastructure Upgrading
WATER UTILITIES CORPORATION TERMS OF REFERENCE FOR THE ENGINEERING DESIGN AND TENDER DOCUMENTATION FOR FRANCISTOWN WATER SUPPLY INFRASTRUCTURE UPGRADING, TENDER No. WUC xxx (2019) APRIL 2019 TABLE OF CONTENTS 1 INTRODUCTION ................................................................................................................... 2 2 PURPOSE OF THE PROJECT ............................................................................................. 4 3 OBJECTIVES OF THE SERVICE ......................................................................................... 5 4 BACKGROUND OF PROJECT AREA ................................................................................. 5 5 CURRENT SITUATION....................................................................................................... 10 6 PREVIOUS STUDIES RELATED TO THE PROJECT AREA. ............................................ 11 7 SCOPE OF WORK AND DETAILED DESCRIPTION OF TASKS ...................................... 11 8 CONSULTANT QUALIFICATION ....................................................................................... 21 9 PROJECT OFFICE ............................................................................................................. 22 10 OBLIGATIONS OF THE CLIENT .................................................................................... 23 11 OBLIGATIONS OF THE CONSULTANT ......................................................................... 24 12 DELIVERABLES ............................................................................................................ -

Land Suitability Zoning for Ecotourism Planning and Development Of
Land Suitability Zoning for Ecotourism Planning and Development of ANGOR UNIVERSITY Dikgatlhong Dam, Botswana Siljeg, Ante; Cavric, Branko; Siljeg, Silvija; Maric, Ivan; Barada, Mirko Geographica Pannonica DOI: 10.5937/gp23-20633 PRIFYSGOL BANGOR / B Published: 01/06/2019 Publisher's PDF, also known as Version of record Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Siljeg, A., Cavric, B., Siljeg, S., Maric, I., & Barada, M. (2019). Land Suitability Zoning for Ecotourism Planning and Development of Dikgatlhong Dam, Botswana. Geographica Pannonica, 23(2), 76-86. https://doi.org/10.5937/gp23-20633 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 23. Sep. 2021 See discussions, stats, and author profiles for this -

Population of Towns, Villages and Associated Localities
POPULATION OF TOWNS, VILLAGES AND ASSOCIATED LOCATLITIES 2001 POPULATION AND HOUSING CENSUS Published by Central Statistics Office, Private Bag: 0024, Gaborone; BOTSWANA Phone: (267) 3671300, Fax: (267) 3952201 Email: [email protected] Website: www.cso.gov.bw April 2002 2001 Botswana Population and Housing Census Page:2 a Total Male Female Gaborone (Census District Code = 01) 186,007 91,823 94,184 Localities in Gaborone 186,007 91,823 94,184 001: Kgale View 2,830 1,340 1,490 002: Gaborone West Extension 2 3,509 1,692 1,817 003: Gaborone West Extension 3 5,031 2,576 2,455 004: Gaborone West Extension 4 6,950 3,495 3,455 005: Gaborone West Extension 5 2,264 1,107 1,157 006: Gaborone West Extension 6 4,615 2,270 2,345 007: Gaborone West Extension 7 3,194 1,560 1,634 008: Gaborone West Phase 2 Ext 8 4,167 1,989 2,178 009: Gaborone West Extension 9 783 368 415 010: Gaborone West Extension 10 1,294 610 684 011: Gaborone West Phase 2 Ext 11 3,737 1,666 2,071 012: Gaborone West Phase 2 Ext 12 446 211 235 014: Gaborone West Extension 14 13 12 1 015: Gaborone West Extension 15 935 463 472 016: Gaborone West Extension 16 849 402 447 017: Gaborone West Extension 17 1,796 865 931 018: Gaborone West Extension 18 653 306 347 019: Gaborone West Extension 19 0 0 0 020: Gaborone West Phase 4 Ext 20 2,096 980 1,116 021: Gaborone West Extension 21 918 440 478 022: Gaborone West Phase4 Extn 22 1,102 494 608 023: Gaborone West Extension 23 264 118 146 024: Gaborone West Extension 24 237 100 137 025: Gaborone West Block 9 Ext 25 1,380 627 753 026: Gaborone West -

Daily Hansard 16 March 2021
DAILY YOUR VOICE IN PARLIAMENT THE SECONDSECOND MEETING MEETING OF THEOF SECONDTHE FIFTH SESSION SESSION OF THEOF THEELEVEN TWELFTHTH PARLIAMENT PARLIAMENT TUESDAY 16 MARCH 2021 MIXEDMIXED VERSION VERSION HANSARDHANSARD NO. 193201 DISCLAIMER Unocial Hansard This transcript of Parliamentary proceedings is an unocial version of the Hansard and may contain inaccuracies. It is hereby published for general purposes only. The nal edited version of the Hansard will be published when available and can be obtained from the Assistant Clerk (Editorial). THE NATIONAL ASSEMBLY SPEAKER The Hon. Phandu T. C. Skelemani PH, MP. DEPUTY SPEAKER The Hon. Mabuse M. Pule, MP. (Mochudi East) Clerk of the National Assembly - Ms B. N. Dithapo Deputy Clerk of the National Assembly - Mr L. T. Gaolaolwe Learned Parliamentary Counsel - Ms M. Mokgosi Assistant Clerk (E) - Mr R. Josiah CABINET His Excellency Dr M. E. K. Masisi, MP. - President His Honour S. Tsogwane, MP. (Boteti West) - Vice President Minister for Presidential Affairs, Governance and Public Hon. K. N. S. Morwaeng, MP. (Molepolole South) - Administration Hon. K. T. Mmusi, MP. (Gabane-Mmankgodi) - Minister of Defence, Justice and Security Hon. Dr L. Kwape, MP. (Kanye South) - Minister of International Affairs and Cooperation Hon. E. M. Molale, MP. (Goodhope-Mabule ) - Minister of Local Government and Rural Development Hon. K. S. Gare, MP. (Moshupa-Manyana) - Minister of Agricultural Development and Food Security Minister of Environment, Natural Resources Conservation Hon. P. K. Kereng, MP. (Specially Elected) - and Tourism Hon. Dr E. G. Dikoloti MP. (Mmathethe-Molapowabojang) - Minister of Health and Wellness Hon. T.M. Segokgo, MP. (Tlokweng) - Minister of Transport and Communications Hon. -

North East District-2011 Population and Housing
NORTH EAST DISTRICT POPULATION AND HOUSING CENsus 2011 SELECTED INDICATORS VOL 7.0 ISBN: 978-99968-463-1-1 NORTH EAST DISTRICT Population and Housing Census 2011 Selected Indicators for Villages and Localities NORTH EAST DISTRICT Population And Housing Census 2011: Selected Indicators For Villages And Localities VOL 7.0 Published by STATISTICS BOTSWANA Private Bag 0024, Gaborone Phone: (267)3671300, Fax: (267) 3952201 Email: [email protected] Website: www.cso.gov.bw COPYRIGHT RESERVED Extracts may be published if source is duly acknowledged ISBN: 978-99968-463-1-1 Table of Contents Preface 3 1.0 Background and Commentary 6 1.1 Background to the Report 6 1.2 Importance of the Report 6 2.0 Population Distribution 6 2.1 Population Age Structure 7 2.2 Labour Force 7 2.3 The Youth 7 2.4 The Elderly 7 3.0 Language 7 4.0 Religious Affiliation 7 5.0 Marital Status 8 6.0 Disability 8 7.0 Employment and Unemployment 9 8.0 Household Size 9 9.0 Access to Portable Water 9 10.0 Source of Fuel for Lighting 9 10.1 Source of Fuel for Cooking 10 10.2 Source of Fuel for Heating 10 11.0 Orphan-hood 11 12.0 Projected Population 2011 – 2026 12 Annexes 13 Figure 1: Map of North East District Mbalambi Gungwe Zwenshambe Sekakangwe MapokaMoroka Gambule Jackalas 1 Kgari Kalakamati Masukwane Vukwi Ramokgwebana Masunga Mosojane Letsholathebe Butale Botalaote Tshesebe Sechele Toteng Senyawe Makaleng Mowana Sebina Mambo Themashanga Tsamaya Matenge Masingwaneng Siviya Gulubane Mabudzane Jackalas 2 Matshelagabedi Francistown Shashe-Mooke Tati Siding Matsiloje Shashe Bridge Borotsi Matopi Ditladi Patayamatebele LEGEND Prepared by Cartographic Unit statistics Botswana, Gaborone 2015 ! Villages NORTH EAST ± Roads DISTRICT District Boundary KM 0 4 8 16 24 32 2 Population and Housing Census 2011 [Selected Indicators] North East District Preface This report follows our strategic resolve to disaggregate the 2011 Population and Housing Census report, and many of our statistical outputs, to cater for specific data needs of users. -
Poverty Mapping 2010
Poverty Global Practice Africa Region May 2015 Mapping Poverty in Botswana 2010 Document of the World Bank ISBN 978-99968-429-9-3 2 Mapping Poverty in Botswana 2010 Statistics Botswana Statistics Botswana Mapping Poverty in Botswana 2010 3 Abbreviations and Acronyms Contents 1 Introduction .......................................................................................................................................................................... 9 BCWIS Botswana Core Welfare Indicator Survey DECRG Development Research Group 2 Poverty measurement in Botswana ..................................................................................................................................10 EA Enumeration Area 2.1 Consumption aggregate....................................................................................................................................................10 ELL Elbers, Lanjouw and Lanjouw 2.2 Data source ........................................................................................................................................................................10 HIES Household Income and Expenditure Survey 2.3 An Overview of Botswana’s Poverty...................................................................................................................................10 PDL Poverty Datum Line PSU Primary Sample Unit 3 Poverty Mapping Method..................................................................................................................................................11 -

Land Suitability Zoning for Ecotourism Planning and Development of Dikgatlhong Dam, Botswana
ISSN 0354-8724 (hard copy) | ISSN 1820-7138 (online) Land Suitability Zoning for Ecotourism Planning and Development of Dikgatlhong Dam, Botswana Ante ŠiljegA, Branko CavrićB, Silvija ŠiljegA, Ivan MarićA, Mirko BaradaA* Received: February 19, 2019 | Revised: April 22, 2019 | Accepted: April 25, 2019 DOI: 10.5937/gp23-20633 Abstract The main objective of this paper was to discuss applications of GIS based multi-criteria decision analysis (GIS MCDA) and Analytical Hierarchy Process (AHP). These two techniques were applied in order to as- sist preparation of the Tourism Management Plan, depicting the most suitable zones for ecotourism de- velopment in Dikgathlong Dam Lease Area (DDLA) as one of the largest resources of potable water in Botswana. The MCDA was based on geo-morphometric, hydrologic, landscape and community indica- tors and criteria which emanated from expert’s opinions, intensive field survey and literature review. In addition the AHP has helped to calculate individual criteria weights and to point the degree of suitabil- ity zones classified as highly suitable, moderately suitable, marginally suitable and not suitable for eco- tourism. After performing both processes and establishing broad management zones it has been found that the Sustainable Development Scenario is the most appropriate option as the future ecotourism development proposal. This research provides new methodology that can be incorporated into future tourism policies and management strategies. Keywords: GIS, multi-criteria decision analysis (MCDA), analytical -

Report of the Delimitation Commission, 2012
REPORT OF THE DELIMITATION COMMISSION, 2012 Delimitation Commission Private Bag 00284 Gaborone 11th February, 2013 His Excellency Lt. Gen. Seretse Khama Ian Khama President of the Republic of Botswana Office of the President Gaborone Your Excellency, DELIMITATION COMMISSION, 2012 We were appointed in terms of Section 64 (1) of the Constitution of Botswana by the Judicial Service Commission on 19th June, 2012 to delimit constituencies following a comprehensive National Population Census conducted in 2011. We have the pleasure and honour to inform Your Excellency that we have delimited the constituencies and now submit our report. Yours faithfully, TABLE OF CONTENTS Acknowledgements................................................................................................. i-ii Introduction.............................................................................................................. iii • General Background......................................................................................... iii • Legal Background.............................................................................................. iii-v • Constraints.......................................................................................................... vi • Methodology....................................................................................................... vi-viii • Naming of Constituencies .............................................................................. viii-x • Observations......................................................................................................