SOTALOL 1. Product Name 2. Qualitative and Quantitative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Management of Chronic Problems
MANAGEMENT OF CHRONIC PROBLEMS INTERACTIONS BETWEEN ALCOHOL AND DRUGS A. Leary,* T. MacDonald† SUMMARY concerned. Alcohol may alter the effects of the drug; drug In western society alcohol consumption is common as is may change the effects of alcohol; or both may occur. the use of therapeutic drugs. It is not surprising therefore The interaction between alcohol and drug may be that concomitant use of these should occur frequently. The pharmacokinetic, with altered absorption, metabolism or consequences of this combination vary with the dose of elimination of the drug, alcohol or both.2 Alcohol may drug, the amount of alcohol taken, the mode of affect drug pharmacokinetics by altering gastric emptying administration and the pharmacological effects of the drug or liver metabolism. Drugs may affect alcohol kinetics by concerned. Interactions may be pharmacokinetic or altering gastric emptying or inhibiting gastric alcohol pharmacodynamic, and while coincidental use of alcohol dehydrogenase (ADH).3 This may lead to altered tissue may affect the metabolism or action of a drug, a drug may concentrations of one or both agents, with resultant toxicity. equally affect the metabolism or action of alcohol. Alcohol- The results of concomitant use may also be principally drug interactions may differ with acute and chronic alcohol pharmacodynamic, with combined alcohol and drug effects ingestion, particularly where toxicity is due to a metabolite occurring at the receptor level without important changes rather than the parent drug. There is both inter- and intra- in plasma concentration of either. Some interactions have individual variation in the response to concomitant drug both kinetic and dynamic components and, where this is and alcohol use. -

Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter And
J Am Board Fam Med: first published as 10.3122/jabfm.2011.01.080096 on 5 January 2011. Downloaded from CLINICAL REVIEW Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter and Fibrillation Madhuri Nair, MD, Lekha K. George, MD, and Santhosh K. G. Koshy, MD This article reviews the safety and efficacy of ibutilide for use in patients with atrial fibrillation and flut- ter. Ibutilide, a class III antiarrhythmic agent, is primarily used for conversion of atrial flutter and fi- brillation and is a good alternative to electrical cardioversion. Ibutilide has a conversion rate of up to 75% to 80% in recent-onset atrial fibrillation and flutter; the conversion rate is higher for atrial flutter than for atrial fibrillation. It is also safe in the conversion of chronic atrial fibrillation/flutter among patients receiving oral amiodarone therapy. Ibutilide pretreatment facilitates transthoracic defibrilla- tion and decreases the energy requirement of electrical cardioversion by both monophasic and biphasic shocks. Pretreatment with ibutilide before electrical defibrillation has a conversion rate of 100% com- pared with 72% with no pretreatment. Ibutilide is also safe and efficient in the treatment of atrial fibril- lation in patients who have had cardiac surgery, and in accessory pathway–mediated atrial fibrillation where the conversion rate of ibutilide is as high as 95%. There is up to a 4% risk of torsade de pointes and a 4.9% risk of monomorphic ventricular tachycardia. Hence, close monitoring in an intensive care unit setting is warranted during and at least for 4 hours after drug infusion. The anticoagulation strat- egy is the same as for any other mode of cardioversion.(J Am Board Fam Med 2011;24:86–92.) Keywords: Antiarrhythmics, Arrhythmia, Atrial Fibrillation, Cardiovascular Disorders, Cardioversion, Drug Ther- copyright. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology
Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology 701 Broad Street, Suite 411 Sewickley, PA 15143 (412) 749-7160 Fax: (412) 749-7388 http://www.heritagevalley.org/sharonrosemanmd Patient Drug Education for Diltiazem / Nifedipine Ointment Diltiazem/Nifedipine ointment is used to help heal anal fissures. The ointment relaxes the smooth muscle around the anus and promotes blood flow which helps heal the fissure (tear). The ointment reduces anal canal pressure, which diminishes pain and spasm. We use a diluted concentration of Diltiazem/Nifedipine compared to what is typically used for heart patients, and this is why you need to obtain the medication from a pharmacy which will compound your prescription. It is also prescribed to treat anal sphincter spasm, painful hemorrhoids and pelvic floor spasm. The Diltiazem/Nifedipine ointment should be applied 3 times per day, or as directed. A pea-sized drop should be placed on the tip of your finger and then gently placed inside the anus. The finger should be inserted 1/3 – 1/2 its length and may be covered with a plastic glove or finger cot. You may use Vaseline ® to help coat the finger or dilute the ointment. (If you are unable or hesitant to use your finger to administer the ointment TELL U S and we will order you a suppository to use as an “applicator”.) If you are advised to mix the Diltiazem/Nifedipine with steroid ointment, limit the steroids to one to two weeks. The first few applications should be taken lying down, as mild light- headedness or a brief headache may occur. -

Drugs and Life-Threatening Ventricular Arrhythmia Risk: Results from the DARE Study Cohort
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2017-016627 on 16 October 2017. Downloaded from Drugs and life-threatening ventricular arrhythmia risk: results from the DARE study cohort Abigail L Coughtrie,1,2 Elijah R Behr,3,4 Deborah Layton,1,2 Vanessa Marshall,1 A John Camm,3,4,5 Saad A W Shakir1,2 To cite: Coughtrie AL, Behr ER, ABSTRACT Strengths and limitations of this study Layton D, et al. Drugs and Objectives To establish a unique sample of proarrhythmia life-threatening ventricular cases, determine the characteristics of cases and estimate ► The Drug-induced Arrhythmia Risk Evaluation study arrhythmia risk: results from the the contribution of individual drugs to the incidence of DARE study cohort. BMJ Open has allowed the development of a cohort of cases of proarrhythmia within these cases. 2017;7:e016627. doi:10.1136/ proarrhythmia. Setting Suspected proarrhythmia cases were referred bmjopen-2017-016627 ► These cases have provided crucial safety by cardiologists across England between 2003 and 2011. information, as well as underlying clinical and ► Prepublication history for Information on demography, symptoms, prior medical and genetic data. this paper is available online. drug histories and data from hospital notes were collected. ► Only patients who did not die as a result of the To view these files please visit Participants Two expert cardiologists reviewed data the journal online (http:// dx. doi. proarrhythmia could be included. for 293 referred cases: 130 were included. Inclusion org/ 10. 1136/ bmjopen- 2017- ► Referral of cases by cardiologists alone may have criteria were new onset or exacerbation of pre-existing 016627). -

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief (if Notes / Similar NCF Active? available) Miscellaneous Medications Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized solution Beta2 Agonist Yes Albuterol, metered dose inhaler Beta2 Agonist NPTC Meeting Update *Any product* Yes (MDI) (Nov 2017) Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Inhibitor Gout (2016) Yes Alogliptin Antidiabetic Agent, Dipeptidyl Peptidase 4 (DPP-4) Inhibitor DPP-IV Inhibitors (2019) Yes Anastrozole Antineoplastic Agent, Aromatase Inhibitor Yes Aspirin Antiplatelet Agent; Nonsteroidal Anti-Inflammatory Drug; Salicylate Yes Azithromycin Antibiotic, Macrolide STIs - PART 1 (2021) Yes Calcium Electrolyte supplement *Any formulation* Yes Carbidopa-Levodopa (immediate Anti-Parkinson Agent; Decarboxylase Inhibitor-Dopamine Precursor Parkinson's Disease Yes release) (2019) Clindamycin, topical ===REMOVED from NCF=== (See Benzoyl Peroxide AND Removed January No Clindamycin, topical combination) 2020 Corticosteroid, intranasal Intranasal Corticosteroid *Any product* Yes Cyanocobalamin (Vitamin B12), Vitamin, Water Soluble Hematologic Supplements Yes oral (2016) Printed on 09/25/2021 Page 1 of 18 National Core Formulary; Last Updated: 09/23/2021 Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
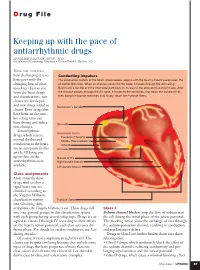
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

An Electrocardiographic Series of Flecainide Toxicity
Indian Pacing and Electrophysiology Journal xxx (xxxx) xxx Contents lists available at ScienceDirect Indian Pacing and Electrophysiology Journal journal homepage: www.elsevier.com/locate/IPEJ An electrocardiographic series of flecainide toxicity * Alexandra Smith , Gregg Gerasimon San Antonio Military Medical Center, Electrophysiology Division, Cardiology Section, 3551 Roger Brooke Drive, San Antonio, TX, 78234, USA article info abstract Article history: Anti-arrhythmic drugs (AADs) uniquely affect the various electrolyte channels in the heart and can slow Received 16 October 2018 conduction, increase refractoriness, and/or decrease automaticity with the goal of preventing tachyar- Received in revised form rhythmias. Due to these properties, these same drugs are by nature pro-arrhythmic. Vaughan-Williams 8 November 2018 classification Ic AADs belong to a class of medications that inhibit sodium channels, leading to decreased Accepted 27 November 2018 conduction velocity of myocytes and Purkinje fibers as well as to decreased automaticity of pacemaker Available online xxx cells. When present in toxic amounts, this leads to classic changes on the electrocardiogram (ECG) that are harbingers of potentially lethal arrhythmias. Presented is a clinical series of ECGs that occurred in a Keywords: fl Flecainide patient who presented with ecainide toxicity. © Antiarrhythmic drugs Copyright 2018, Indian Heart Rhythm Society. Production and hosting by Elsevier B.V. This is an open Toxicity access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Atrial fibrillation Flecainide toxicity can lead to bradycardia, sinoatrial block, and Abbreviations asystole, as well as to first and second degree atrioventricular block. Sinus bradycardia is more common in patients with pre-existing AAD Antiarrhythmic drug sinus node dysfunction [2].