Atrial Fibrillation in the Surgical Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Use of Intravenous Esmolol to Predict Efficacy of Oral Beta-Adrenergic Blocker Therapy in Patients with Neurocardiogenic Syncope
402 JACC Vol. 19, No. 2 February 1992:402-8 REPORTS ON THERAPY Use of Intravenous Esmolol to Predict Efficacy of Oral Beta Adrenergic Blocker Therapy in Patients With Neurocardiogenic Syncope JASBIR S. SRA, MD, VISHNUBHAKTA S. MURTHY, MD, PHD, MOHAMMAD R. JAZAYERI, MD, FACC, YUE-HUA SHEN, MD, PAUL J. TROUP, MD, FACC, BOAZ AVITALL, MD, PHD, MASOOD AKHTAR, MD, FACC Milwaukee, Wisconsin The usefulness of esmolol in predicting the efficacy of treatment Irrespective of their response to esmolol infusion, all patients with an oral beta-adrenergic blocking agent was evaluated in 27 had a follow-up tilt test with oral metoprolol after an interval of consecutive patients with neurocardiogenic syncope. Seventeen ~5 half-lives of the drug. All 16 patients (100%) with a negative patients had a positive head-up tilt test response at baseline and 10 tilt test response during esmolol infusion had a negative tilt test patients required intravenous isoproterenol for provocation of response with oral metoprolol. Of the 11 patients with a positive hypotension. All patients were then given a continuous esmolol tilt test response during esmolol infusion, 10 (90%) continued to infusion (500 JLg/kg per min loading dose for 3 min followed by have a positive response with oral metoprolol. 300 Jlg/kg per min maintenance dose) and rechallenged with a It is concluded that in the electrophysiology laboratory, es head-up tilt test at baseline or with isoproterenol. molol can accurately predict the outcome of a head-up tilt Of the 17 patients with a positive baseline tilt test response, 11 response to oral metoprolol. -
Esmolol Hydrochloride 10 Mg/Ml Solution for Injection Esmolol Hydrochloride
Package leaflet: Information for the patient Esmolol hydrochloride 10 mg/ml solution for injection Esmolol hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. – Keep this leaflet. You may need to read it again. – If you have any further questions, ask your doctor or nurse. – This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. – If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Esmolol hydrochloride 10 mg/ml solution for injection is and what it is used for 2. What you need to know before you use Esmolol hydrochloride 10 mg/ml solution for injection 3. How to use Esmolol hydrochloride 10 mg/ml solution for injection 4. Possible side effects 5. How to store Esmolol hydrochloride 10 mg/ml solution for injection 6. Contents of the pack and other information 1. What Esmolol hydrochloride 10 mg/ml chlor promazine, used to treat mental dis - solution for injection is and what it is orders) used for – Clozapine which is used to treat mental Esmolol hydrochloride 10 mg/ml belongs to the disorders group of beta-blockers. These medicines slow – Epinephrine, which is used to treat allergic down the heartbeat and reduce blood pressure. reactions – Medicines used to treat asthma Esmolol hydrochloride 10 mg/ml is used for – Medicines used to treat colds or a blocked short-term treatment if your heart beats too nose, called nasal “decongestants” fast. -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology
Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology 701 Broad Street, Suite 411 Sewickley, PA 15143 (412) 749-7160 Fax: (412) 749-7388 http://www.heritagevalley.org/sharonrosemanmd Patient Drug Education for Diltiazem / Nifedipine Ointment Diltiazem/Nifedipine ointment is used to help heal anal fissures. The ointment relaxes the smooth muscle around the anus and promotes blood flow which helps heal the fissure (tear). The ointment reduces anal canal pressure, which diminishes pain and spasm. We use a diluted concentration of Diltiazem/Nifedipine compared to what is typically used for heart patients, and this is why you need to obtain the medication from a pharmacy which will compound your prescription. It is also prescribed to treat anal sphincter spasm, painful hemorrhoids and pelvic floor spasm. The Diltiazem/Nifedipine ointment should be applied 3 times per day, or as directed. A pea-sized drop should be placed on the tip of your finger and then gently placed inside the anus. The finger should be inserted 1/3 – 1/2 its length and may be covered with a plastic glove or finger cot. You may use Vaseline ® to help coat the finger or dilute the ointment. (If you are unable or hesitant to use your finger to administer the ointment TELL U S and we will order you a suppository to use as an “applicator”.) If you are advised to mix the Diltiazem/Nifedipine with steroid ointment, limit the steroids to one to two weeks. The first few applications should be taken lying down, as mild light- headedness or a brief headache may occur. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
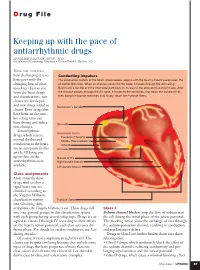
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Comparative Study of IV Esmolol, IV Diltiazem, and IV Lignocaine Hydrochloride in Attenuating Pressure Response to Laryngoscopy and Intubation
Jemds.com Original Research Article Comparative Study of IV Esmolol, IV Diltiazem, and IV Lignocaine Hydrochloride in Attenuating Pressure Response to Laryngoscopy and Intubation Sathya Narayanan Karunanithi1, Geeta Bhandari2, Kedar Singh Shahi3, Aditya Kumar Chauhan4, Pooja Gautam5 1Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 2 Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 3Department of Surgery, Government Medical College, Haldwani, Uttarakhand, India. 4Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 5Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. ABSTRACT BACKGROUND Not many studies have compared more than two drugs in attenuating pressor Corresponding Author: responses to laryngoscopy and intubation. This study compares four groups of Dr. Geeta Bhandari, considerable size. The present study compared intravenous esmolol, diltiazem, and Professor and HOD, Department of Anaesthesiology, lignocaine, for their efficacy to abate pressure response to laryngoscopy and Critical Care, Pain and Palliative Medicine, intubation. Government Medical College, Haldwani-263139, Uttarakhand, India. METHODS E-mail: [email protected] This is a prospective, randomized, double-blinded, controlled clinical study conducted among 220 patients of ASA grade I/II (age 18–60 years), undergoing DOI: 10.14260/jemds/2020/447 elective surgical procedure requiring general anaesthesia with endotracheal intubation over a period of 15 months at a tertiary hospital setup. Study subjects How to Cite This Article: were categorised as Groups D, E, L, and N that received diltiazem (0.2 mg/Kg IV), Karunanithi SN, Bhandari G, Shahi KS, et esmolol (2 mg/Kg IV), lignocaine (1.5 mg/Kg IV), and normal saline, respectively; al. -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -

Oral Calcium Channel Blocker Comparison
Oral Calcium Channel Blocker Comparison Various calcium channel blockers (CCBs) have been periodically shorted. Below is a table of dosing comparisons. General notes: No dose equivalencies among the CCBs have been established; estimate an approximate dose using the dosing ranges. The contraindications and adverse effects of non-dihydropyridine (DHP) CCBs (diltiazem and verapamil) are quite different from DHP CCBs (amlodipine, felodipine, nifedipine). Consider staying with the same type of CCB if possible unless other considerations warrant changing types. Be sure to check for drug interactions if switching agents. Calcium Channel Blocker Comparisons1,2 Doses CCB Contraindications Hypertension Stable angina DHP Adverse Effects: pedal edema, flushing, palpitations, headache Nifedipine MR 30-60 mg up to 90 mg daily 2.5-5 mg to 10 mg Amlodipine 5-10 mg daily daily severe aortic stenosis 2.5-10 mg to May be useful but Felodipine 20 mg daily not indicated Non-DHP Adverse Effects: angina, heart failure; constipation, especially with verapamil 120-240 mg post myocardial infarction with 120-180 mg to 360 Diltiazem MR to 360 mg ejection fraction (EF) <40% mg daily daily 2nd or 3rd degree AV block, or sick sinus syndrome (unless functioning ventricular pacemaker) atrial flutter/atrial fibrillation and accessory bypass tract (e.g. Wolff- 80-240 mg Parkinson-White syndrome, Lown- 180 mg to 480 mg once daily to Ganong-Levine syndrome) Verapamil MR daily in one or two 180-240 mg combination with ivabradine doses BID Verapamil extreme bradycardia severe heart failure and or EF<40% combination with drugs that affect cardiac conduction CCB= calcium channel blocker; DHP= dihydropyridine; MR=modified release such as XL, CD, SR, etc. -

Comparison of the Β-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacoc
1521-0103/359/1/73–81$25.00 http://dx.doi.org/10.1124/jpet.116.232884 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 359:73–81, October 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Comparison of the b-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions Shahrooz Nasrollahi-Shirazi, Sonja Sucic, Qiong Yang, Michael Freissmuth, and Christian Nanoff Institute of Pharmacology, Center of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria Received February 15, 2016; accepted July 18, 2016 Downloaded from ABSTRACT Blockage of b1-adrenergic receptors is one of the most effective was very low. Both landiolol and esmolol caused a very modest treatments in cardiovascular medicine. Esmolol was introduced rise in cAMP levels but a robust increase in the phosphorylation some three decades ago as a short-acting b1-selective antag- of extracellular signal regulated kinases 1 and 2, indicating that the onist. Landiolol is a more recent addition. Here we compared the two drugs exerted partial agonist activity with a signaling bias. If two compounds for their selectivity for b1-adrenergic receptors cells were incubated for $24 hours in the presence of $1 mM jpet.aspetjournals.org over b2-adrenergic receptors, partial agonistic activity, signaling esmolol, the levels of b1-adrenergic—but not of b2-adrenergic— bias, and pharmacochaperoning action by using human embry- receptors increased. This effect was contingent on export of onic kidney (HEK)293 cell lines, which heterologously express the b1-receptor from endoplasmic reticulum and was not seen each human receptor subtype.