Focusing on Your Needs with Platform Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Final Stakeholder List PDF 190 KB
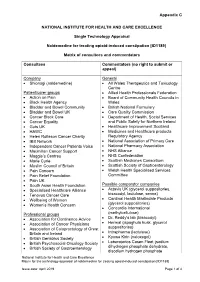
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Formulation of Poloxamers for Drug Delivery
Journal of Functional Biomaterials Review Formulation of Poloxamers for Drug Delivery Andrew M. Bodratti and Paschalis Alexandridis * Department of Chemical and Biological Engineering, University at Buffalo, The State University of New York (SUNY), Buffalo, NY 14260, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-716-645-1183 Received: 18 December 2017; Accepted: 14 January 2018; Published: 18 January 2018 Abstract: Poloxamers, also known as Pluronics®, are block copolymers of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), which have an amphiphilic character and useful association and adsorption properties emanating from this. Poloxamers find use in many applications that require solubilization or stabilization of compounds and also have notable physiological properties, including low toxicity. Accordingly, poloxamers serve well as excipients for pharmaceuticals. Current challenges facing nanomedicine revolve around the transport of typically water-insoluble drugs throughout the body, followed by targeted delivery. Judicious design of drug delivery systems leads to improved bioavailability, patient compliance and therapeutic outcomes. The rich phase behavior (micelles, hydrogels, lyotropic liquid crystals, etc.) of poloxamers makes them amenable to multiple types of processing and various product forms. In this review, we first present the general solution behavior of poloxamers, focusing on their self-assembly properties. This is followed by a discussion of how the self-assembly properties of poloxamers can be leveraged to encapsulate drugs using an array of processing techniques including direct solubilization, solvent displacement methods, emulsification and preparation of kinetically-frozen nanoparticles. Finally, we conclude with a summary and perspective. Keywords: Pluronic; poly(ethylene oxide); poly(ethylene glycol); nanomedicine; excipient; formulation; solubilization; anticancer; micelle; nanoparticle; hydrogel 1. -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

Poloxamer Hydrogels for Biomedical Applications
pharmaceutics Review Poloxamer Hydrogels for Biomedical Applications Eleonora Russo * and Carla Villa Department of Pharmacy, University of Genoa, Viale Benedetto XV, 16132 Genova, Italy; [email protected] * Correspondence: [email protected] Received: 31 October 2019; Accepted: 6 December 2019; Published: 10 December 2019 Abstract: This review article focuses on thermoresponsive hydrogels consisting of poloxamers which are of high interest for biomedical application especially in drug delivery for ophthalmic, injectable, transdermal, and vaginal administration. These hydrogels remain fluid at room temperature but become more viscous gel once they are exposed to body temperature. In this way, the gelling system remains at the topical level for a long time and the drug release is controlled and prolonged. Poloxamers are synthetic triblock copolymers of poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO), also commercially known as Pluronics®, Synperonics® or Lutrol®. The different poloxamers cover a range of liquids, pastes, and solids, with molecular weights and ethylene oxide–propylene oxide weight ratios varying from 1100 to 14,000 and 1:9 to 8:2, respectively. Concentrated aqueous solutions of poloxamers form thermoreversible gels. In recent years this type of gel has arouse interest for tissue engineering. Finally, the use of poloxamers as biosurfactants is evaluated since they are able to form micelles in an aqueous environment above a concentration threshold known as critical micelle concentration (CMC). This property is exploited for drug delivery and different therapeutic applications. Keywords: poloxamer; hydrogels; micelle; thermosensitive; biomedical; copolymer 1. Introduction The word “hydrogel”, according to Lee, Kwon and Park is due to an article published in 1894, but the first crosslinked network material that appeared in literature and that has been described by its typical hydrogel properties, was a polyhydroxyethylmethacrylate (HEMA) hydrogel developed much later, in 1960, by O. -

Early-Stage Atherosclerosis in Poloxamer 407
Korolenko et al. Lipids in Health and Disease (2016) 15:16 DOI 10.1186/s12944-016-0186-7 RESEARCH Open Access Early-stage atherosclerosis in poloxamer 407-induced hyperlipidemic mice: pathological features and changes in the lipid composition of serum lipoprotein fractions and subfractions Tatyana A. Korolenko1, Thomas P. Johnston2*, Fedor V. Tuzikov3,4, Natalia A. Tuzikova3,4, Alexandr B. Pupyshev6, Victor K. Spiridonov1, Natalya V. Goncharova1, Igor V. Maiborodin7 and Natalia A. Zhukova5 Abstract Background: The aims of this study were to evaluate the effect of poloxamer 407 administration on atherogenic serum lipoprotein fractions and subfractions associated with cholesterol, triglycerides and phospholipids, as well as the onset of early atherosclerosis, in mice. Methods: Mice were administered either sterile saline or poloxamer 407 (to induce a dose-controlled hyperlipidemia) for 1 month and then sacrificed at 1, 4 and 10 days after the last dose of poloxamer 407. Systolic and diastolic blood pressure, the activity of a cysteine protease (cathepsin B) in cardiac and liver tissue, and histological/morphological examination of heart and liver specimens was performed for each group of mice at each time point. Lastly, small angle X-ray scattering was utilized to analyze the lipoprotein fractions and subfractions associated with cholesterol, triglycerides and phospholipids for both groups of mice at each time point. Statistical analysis was performed using one-way, analysis-of-variance with post hoc analysis to determine significantly different mean values, while correlation analysis employed the Spearman test. Results: Poloxamer 407-treated mice revealed significant hyperlipidemia, moderately elevated blood pressure, general lipidosis in liver cells, increased cysteine protease activity in heart tissue, and contractile-type changes in cardiomyocytes. -

WO 2016/095083 Al O
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/095083 Al 23 June 2016 (23.06.2016) P O P CT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/20 (2006.01) A61K 8/19 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K /27 (2006.01) A61Q 11/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/CN2014/093826 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, 15 December 2014 (15. 12.2014) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: COLGATE-PALMOLIVE COMPANY (84) Designated States (unless otherwise indicated, for every [US/US]; 300 Park Avenue, New York 10022 (US). kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (72) Inventor; and TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant (for SC only): XV, Yun [US/CN]; 338 Qingni- TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, an Road, Xingang Huangpu, GETDD, Guangzhou, Guang DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, dong 510730 (CN). -

Drugs Used in Treating Constipation and IBS
Drugs used in treating constipation and IBS Define constipation Know the different symptoms of constipation Know the different lines of treatment of constipation Identify the different types of laxatives Discuss the pharmacokinetics, dynamics, side effects and uses of laxatives Discuss the difference between different treatment including bulk forming laxatives, osmotic laxatives, stimulant laxatives And stool softeners (lubricants). Define bowel syndrome (IBS). Identify the pharmacokinetics, dynamics, side effects and uses of drugs used for IBS. extra information and further explanation important doctors notes Drugs names Mnemonics Kindly check the editing file before studying this document constipation and IBS Very useful. Don’t miss it Infrequent defecation, often with straining and the passage of hard, uncomfortable stools. - May be accompanied by other symptoms: Abdominal discomfort and rectal pain, Flatulence, Loss of appetite, Lethargy & Depression. Decreased motility in colon: Decrease in water and fiber Difficulty in evacuation: Drug-induced: contents of diet. 1. Local painful conditions: 1. Anticholinergic agents “Unbalanced diet” anal fissures, piles. 2. Opioids 2. Lack of muscular 3. Iron exercise. 4. Antipsychotics. 5. Anti-depressant, anti- histamine, has anticholinergic effect Adequate fluid intake High fiber contents in diet Use drugs (laxatives or Regulation of bowel habit. purgatives more watery effect) Regular exercise Avoid drugs causing constipation. • Drugs that hasten the transit of food through the gastrointestinal tract are called (Or we say the drugs that increase GI motility) (محركات) or purgatives(ملينات) laxatives • Loosen stools and increase bowel movement Bulk forming laxatives (increase the size of stool ) • Increase volume of non-absorbable solid residue. Osmotic laxatives (cause water withdrawal by sugar or salt) • Increase water content in large intestine. -

Managing Opioid-Induced Constipation
Visual summary MODIFY PAIN RELIEF Managing opioid-induced constipation Is the current dose of opioid needed to control pain? Constipation affects many patients using opioids Could pain be relieved in other ways? to relieve pain associated with advanced cancer Non-opioid analgesics Interventional pain management or terminal disease. Constipation is often multifactorial in such patients, Could an opioid with less constipating effect be used? and opioids may be one of several causes. As well Buprenorphine Transdermal fentanyl as direct treatment of the constipation, adjusting medication regimens and treating exacerbating factors may help to provide relief from bowel transit Is a referral to other specialists needed? symptoms. Palliative care Neuro-gastroenterology Pain clinic ADDRESS EXACERBATING FACTORS DIRECTLY TREAT CONSTIPATION Drugs Non-pharmacological options 5HT3 antagonists Anticholinergics Opioids Increase fluid consumption Increase dietary fibres Antipsychotics Diuretics Iron Increase physical activity (within patients’ capabilities) Calcium supplements Calcium channel blockers Privacy and comfort during defecation Chemotherapies Complementary therapy Thalidomide Vinca alkaloids Positioning on toilet (to relax the puborectalis muscle) knees higher than hips, leaning forward Busulfan Carboplatin with elbows on knees, straightened spine Manual removal Nutritional and if the constipation is severe and refractory to other therapies metabolic issues Pharmacological management Dehydration Reduced food and fluids intake Oral laxatives Poor -

Treatment of Faecal Impaction in Children Using Combined
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Groningen University of Groningen Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate Lamanna, Anthony; Dughetti, Lauren D.; Jordan-Ely, Julie A.; Dobson, Kyla M.; Dynan, Megan; Foo, Adeline; Kooiman, Louise M. P.; Murakami, Naomi; Fiuza, Kaic; Foroughi, Siavash Published in: BJGP open DOI: 10.1002/jgh3.12062 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2018 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Lamanna, A., Dughetti, L. D., Jordan-Ely, J. A., Dobson, K. M., Dynan, M., Foo, A., ... Southwell, B. R. (2018). Treatment of fecal impaction in children using combined polyethylene glycol and sodium picosulphate. BJGP open, 2(4), 144-151. https://doi.org/10.1002/jgh3.12062 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. -

Compound Macrogol Oral Powder Sugar Free. Please Tell Your Doctor Or Pharmacist If You Are Taking, Have Recently Taken Or Might Take Any Other Medicines
Package leaflet: Information for the user Compound Macrogol Oral Powder Sugar Free Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. - Keep this leaflet. You may need to read it again. - Ask your pharmacist if you need more information or advice. - If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. - You must talk to a doctor if you do not feel better or if you feel worse after 14 days for chronic constipation, or after 3 days for faecal impaction. What is in this leaflet: 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for 2. What you need to know before you take Compound Macrogol Oral Powder Sugar Free 3. How to take Compound Macrogol Oral Powder Sugar Free 4. Possible side effects 5. How to store Compound Macrogol Oral Powder Sugar Free 6. Contents of the pack and other information 1. What Compound Macrogol Oral Powder Sugar Free is and what it is used for Compound Macrogol Oral Powder Sugar Free contains macrogol 3350 and the electrolytes sodium chloride, sodium hydrogen carbonate and potassium chloride. Macrogol 3350 is a laxative used for the treatment of constipation, especially if you have been constipated for a long time. It is also used to treat a build up of hard faeces in your bowel which may be a result of long-term constipation (this is known as faecal impaction).