Control of Functional Connectivity in Cerebral Cortex by Basal Ganglia Mediated Synchronization • Pouzzner
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
ACTIONS OF ISOVALINE AND ENDOGENOUS AMINO ACIDS ON INHIBITORY RECEPTORS IN VENTROBASAL THALAMUS by JAMES EDWARD COOKE BSc, Carleton University, 2002 MSc, Carleton University, 2004 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Pharmacology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) August, 2010 © James Edward Cooke, 2010 ABSTRACT This thesis consists of three manuscripts that examine the effects of amino acids and inhibitory neurotransmission in ventrobasal thalamus, a region of the brain responsible for processing nociceptive information. In the first manuscript we examined the possibility that a proposed antagonist of receptors for endogenous amino acids was selective for -amino acids. In the second manuscript, we determined the ionic mechanism of action of isovaline, a non-biogenic amino acid with chemical similarity to glycine and GABA. In the third manuscript, we determined that the inhibitory action of isovaline is mediated by metabotropic receptors, likely GABAB. In the first manuscript we used whole-cell patch clamp electrophysiology and immunohistochemistry to examine the differential antagonism of GABAAergic IPSCs by a proposed -amino acid antagonist, TAG. In IPSCs that were attributable to both GABAergic and glycinergic stimulation, TAG significantly reduced both components. TAG had no effect in purely GABAAergic IPSCs. Our data supports the hypothesis that a specific GABAA subunit, 4, is sensitive to the -amino acid antagonist, TAG. The second manuscript examines the ionic mechanism of action of isovaline, demonstrated to have analgesic properties in animal models. Isovaline inhibited action potential firing of thalamocortical neurons by activating a long-lasting potassium conductance that was insensitive to the glycine antagonist, strychnine. -

Amygdaloid Projections to the Ventral Striatum in Mice: Direct and Indirect Chemosensory Inputs to the Brain Reward System
ORIGINAL RESEARCH ARTICLE published: 22 August 2011 NEUROANATOMY doi: 10.3389/fnana.2011.00054 Amygdaloid projections to the ventral striatum in mice: direct and indirect chemosensory inputs to the brain reward system Amparo Novejarque1†, Nicolás Gutiérrez-Castellanos2†, Enrique Lanuza2* and Fernando Martínez-García1* 1 Departament de Biologia Funcional i Antropologia Física, Facultat de Ciències Biològiques, Universitat de València, València, Spain 2 Departament de Biologia Cel•lular, Facultat de Ciències Biològiques, Universitat de València, València, Spain Edited by: Rodents constitute good models for studying the neural basis of sociosexual behavior. Agustín González, Universidad Recent findings in mice have revealed the molecular identity of the some pheromonal Complutense de Madrid, Spain molecules triggering intersexual attraction. However, the neural pathways mediating this Reviewed by: Daniel W. Wesson, Case Western basic sociosexual behavior remain elusive. Since previous work indicates that the dopamin- Reserve University, USA ergic tegmento-striatal pathway is not involved in pheromone reward, the present report James L. Goodson, Indiana explores alternative pathways linking the vomeronasal system with the tegmento-striatal University, USA system (the limbic basal ganglia) by means of tract-tracing experiments studying direct *Correspondence: and indirect projections from the chemosensory amygdala to the ventral striato-pallidum. Enrique Lanuza, Departament de Biologia Cel•lular, Facultat de Amygdaloid projections to the nucleus accumbens, olfactory tubercle, and adjoining struc- Ciències Biològiques, Universitat de tures are studied by analyzing the retrograde transport in the amygdala from dextran València, C/Dr. Moliner, 50 ES-46100 amine and fluorogold injections in the ventral striatum, as well as the anterograde labeling Burjassot, València, Spain. found in the ventral striato-pallidum after dextran amine injections in the amygdala. -
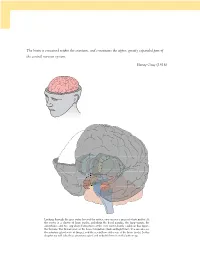
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Traditional Medicine and Ethnomedical Research
International Conference on TRADITIONAL MEDICINE AND ETHNOMEDICAL RESEARCH OCTOBER 24-25, 2019 ToKYO, JAPAN Theme: Healthy Life in Natures Lap Radisson Hotel Narita 286-0221 Chiba Tomisato-shi Nakaei 650-35 Tokyo, Japan @ICTM2019 TraditionalMedicine_EthnoMedicine_2019 ICTM https://traditional-medicine-conferences.magnusgroup.org/ EXHIBITOR 2019 ICTM 2019 BOOK OF ABSTRACTS International Conference on TRADITIONAL MEDICINE AND EtHNOMEDICAL RESEARCH Theme: Healthy Life in Natures Lap October 24-25, 2019 Tokyo, Japan INDEX Contents Pages Welcome Message 8 Keynote Speakers 10 About the Host 11 About Exhibitor 12 Keynote Session (Day 1) 13 Speaker Session (Day 1) 19 Keynote Session (Day 2) 37 Speaker Session ( Day 2) 43 Poster Presentations 66 Attendees Mailing List 74 ICTM 2019 Abuzar Ansari Amrita Sharma Annette Booiman Arturo Huerta-López Baohong Jiang Ewha Womens University Amrita’s Ayuryogavidya, a Mensendieckmoves, Comunidad Biocultural, A.C., Shanghai Institute of Materia Mokdong Hospital, Korea centre of excellence for well- Netherlands México Medica, China ness and holistic health, India Barend van Heerden Bernd-Michael Löffler Chanin Sillapachaiyaporn Charles Shang Chen Che-Yi Indian Board of Alternative Institute for Mitochondrial Chulalongkorn University, University Of Texas MD Ander- Taipei Medical University, Medicine, South Africa Medicine, Germany Thailand son Cancer Center, USA Taiwan Chihiro Sugita Dominique de Rocca Serra Fai Chan Fossion Jean G. R. Padmasiri Kyushu University of Health University of Corsica Deli Aroma LLC Group Investigation of dys-Au- University of the Visual and and Welfare, Japan France USA tonomy (GidA), Belgium Performing Arts, Sri Lanka Geoffrey W Abbott Hiroyoshi Tahata Irmgard Rose Parys James Teng Jia-Ming Chen University of California, Japanese Rolfing Association, Community Practice Dres. -

42711 IPEG Programmaboekje
19 th Biennial Conference 19th Biennial Conference October 26th – 30th 2016 October 26 Nijmegen, the Netherlands th – 30 th 2016 Nijmegen, the Netherlands 2016 Nijmegen, 42711 IPEG programmaboekje omslag.indd 1 06-10-16 17:52 The “International Pharmaco-EEG Society, Association for Electrophysiological Brain Research in Preclinical and Clinical Pharmacology and Related Fields” (IPEG) is a non-profit organization, established in 1980 and composed of scientists and researchers actively involved in electrophysiological brain research in preclinical and clinical pharmacology, neurotoxicology and related areas of interest. my.thesis nl for the design of your Thesis & to show your Thesis 42711 IPEG programmaboekje omslag.indd 2 06-10-16 17:52 IPEG 2016 in Nijmegen | 1 Welcome to Nijmegen, dear attendants of the 19th IPEG meeting. Nijmegen, a 2000 year old city; Nijmegen, a Roman and a medieval city; Nijmegen, the home town of the first catholic university funded by the Faithfull to improve education of a suppressed part of the Netherlands; Nijmegen, the city that heavily suffered in WWII; Nijmegen, the home town of the Donders Institute. Nijmegen, as we hope and trust, your home town for the upcoming IPEG meeting. As you all know, electrophysiological brain research has a long tradition going back as far as 1875 when the first report on the animal electroencephalogram (EEG) was published by Caton. The often forgotten Polish physiologist Adolf Beck was also a EEG pioneer many years before Hans Berger’s initial reports. Beck recorded electrical potentials in several brain areas evoked by peripheral sensory stimuli. Using this technique, Beck localised various centres in the brain of several animal species and described desynchronization in electrical brain potentials. -

Functional Connectivity Changes in Cerebral Small
Schulz et al. BMC Medicine (2021) 19:103 https://doi.org/10.1186/s12916-021-01962-1 RESEARCH ARTICLE Open Access Functional connectivity changes in cerebral small vessel disease - a systematic review of the resting-state MRI literature Maximilian Schulz1, Caroline Malherbe1,2, Bastian Cheng1, Götz Thomalla1 and Eckhard Schlemm1* Abstract Background: Cerebral small vessel disease (CSVD) is a common neurological disease present in the ageing population that is associated with an increased risk of dementia and stroke. Damage to white matter tracts compromises the substrate for interneuronal connectivity. Analysing resting-state functional magnetic resonance imaging (fMRI) can reveal dysfunctional patterns of brain connectivity and contribute to explaining the pathophysiology of clinical phenotypes in CSVD. Materials and methods: This systematic review provides an overview of methods and results of recent resting- state functional MRI studies in patients with CSVD. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol, a systematic search of the literature was performed. Results: Of 493 studies that were screened, 44 reports were identified that investigated resting-state fMRI connectivity in the context of cerebral small vessel disease. The risk of bias and heterogeneity of results were moderate to high. Patterns associated with CSVD included disturbed connectivity within and between intrinsic brain networks, in particular the default mode, dorsal attention, frontoparietal control, and salience networks; decoupling of neuronal activity along an anterior–posterior axis; and increases in functional connectivity in the early stage of the disease. Conclusion: The recent literature provides further evidence for a functional disconnection model of cognitive impairment in CSVD. -

19Th Biennial IPEG Meeting Nijmegen, the Netherlands
Neuropsychiatric Electrophysiology 2016, 2(Suppl 1):8 DOI 10.1186/s40810-016-0021-4 MEETINGABSTRACTS Open Access 19th biennial IPEG Meeting Nijmegen, The Netherlands. 26-30 October 2016 Published: 29 November 2016 Training course measures. This will be illustrated by means of pertinent examples. These include elucidating the mechanisms of stimulant action re- A1 mediating deficient impulse control and the role of the cannabinoid Thalamocortical sleep oscillations system in human working memory, as well as drug effects on Igor Timofeev1,2 sensory gating and specific aspects of visual-spatial attention. Other 1Department of Psychiatry and Neuroscience, Université Laval, Québec, examples concern the added sensitivity of EEG and ERP measures, Canada; 2Centre de recherche de l’Institut universitaire en santé mentale relative to that of performance measures, in detecting effects of alco- de Québec (CRIUSMQ), Université Laval, Québec, Canada hol, and more generally in monitoring and predicting vigilance and Neuropsychiatric Electrophysiology 2016, 2(Suppl 1):A1 the ability to detect external signals in the immediate future. Rela- tions between brain signals and cognitive competences are revealed In waking and sleeping states, thalamocortical system generates a by either comparing different individuals, or moment-to-moment variety of oscillations ranging from 0.1 Hz to hundreds of Hz. Most of fluctuations within individuals, or differences in state (e.g., drug- them are present during NREM sleep, but slower activities prevail in induced) within individuals. this state of vigilance. Thalamocortical network is organized in a loop in which thalamocortical cells excite reticular thalamic and neocor- tical cells, reticular thalamic cells inhibit thalamocortical cells and A3 corticothalamic cells excite thalamocortical and reticular thalamic EEG and ERP as key techniques for functional brain alterations cells. -

Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum Along 2 the Dorsal-Ventral Axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.14.876516; this version posted December 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Distinct transcriptomic cell types and neural circuits of the subiculum and prosubiculum along 2 the dorsal-ventral axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E. Hirokawa1, Thuc Nghi Nguyen1, Lucas T. Graybuck1, Olivia 5 Fong1, Phillip Bohn1, Kiet Ngo1, Kimberly A. Smith1, Christof Koch1, John W. Phillips1, Ed S. Lein1, 6 Julie A. Harris1, Bosiljka Tasic1, Hongkui Zeng1 7 8 1Allen Institute for Brain Science, Seattle, WA 98109, USA 9 10 2Lead Contact 11 12 *Correspondence: [email protected] (SLD) 13 14 15 Highlights 16 17 1. 27 transcriptomic cell types identified in and spatially registered to “subicular” regions. 18 2. Anatomic borders of “subicular” regions reliably determined along dorsal-ventral axis. 19 3. Distinct cell types and circuits of full-length subiculum (Sub) and prosubiculum (PS). 20 4. Brain-wide cell-type specific projections of Sub and PS revealed with specific Cre-lines. 21 22 23 In Brief 24 25 Ding et al. show that mouse subiculum and prosubiculum are two distinct regions with differential 26 transcriptomic cell types, subtypes, neural circuits and functional correlation. The former has obvious 27 topographic projections to its main targets while the latter exhibits widespread projections to many 28 subcortical regions associated with reward, emotion, stress and motivation. -

Rehabilitation of Executive Dysfunction in Multiple Sclerosis: Cognitive, Behavioural and Neurophysiological Effects of Goal Management Training
Rehabilitation of Executive Dysfunction in Multiple Sclerosis: Cognitive, Behavioural and Neurophysiological Effects of Goal Management Training by Nadine Marie Richard A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Psychology University of Toronto © Copyright by Nadine Marie Richard 2013 Rehabilitation of Executive Dysfunction in Multiple Sclerosis: Cognitive, Behavioural and Neurophysiological Effects of Goal Management Training Nadine Marie Richard Doctor of Philosophy Department of Psychology University of Toronto 2013 Abstract Multiple sclerosis (MS) is a chronic, progressive central nervous system disease characterized by distributed white matter injury. Particularly prevalent in Canada, MS is a leading cause of disability with extended personal and societal costs given its early onset (on average between 20- 40 years of age). Deficits in executive functioning are common and detrimental to employment, daily functioning and quality of life, however their precise nature remains underspecified. Two studies were undertaken to: (1) describe the executive processes affected in MS, including neurophysiological correlates, and (2) explore, in a double-blind randomized controlled trial, patients’ response to an intervention for improving behavioural self-regulation. Study 1 described significant functional limitations and impairments in processing speed and executive control of attention, memory and working memory in MS patients, both relative to neurologically healthy controls and as a monotonic function of disease severity. Mean amplitudes for event- related potentials (ERPs) including the P3 and the error-related negativity and positivity (ERN, Pe) were linked to behavioural markers of attention control and performance monitoring. ERP amplitudes and ERP-behavioural associations appeared sensitive to the presence and severity of executive dysfunction related to disease progression. -

Reading Speech from Still and Moving Faces: the Neural Substrates of Visible Speech
Reading Speech from Still and Moving Faces: The Neural Substrates of Visible Speech Gemma A. Calvert1 and Ruth Campbell2 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/15/1/57/1757731/089892903321107828.pdf by guest on 18 May 2021 Abstract & Speech is perceived both by ear and by eye. Unlike heard regions including the left inferior frontal (Broca’s) area, left speech, some seen speech gestures can be captured in superior temporal sulcus (STS), and left supramarginal gyrus stilled image sequences. Previous studies have shown that in (the dorsal aspect of Wernicke’s area). Stilled speech hearing people, natural time-varying silent seen speech can sequences also generated activation in the ventral premotor access the auditory cortex (left superior temporal regions). cortex and anterior inferior parietal sulcus bilaterally. Using functional magnetic resonance imaging (fMRI), the Moving faces generated significantly greater cortical activa- present study explored the extent to which this circuitry was tion than stilled face sequences, and in similar regions. activated when seen speech was deprived of its time-varying However, a number of differences between stilled and moving characteristics. speech were also observed. In the visual cortex, stilled faces In the scanner, hearing participants were instructed to generated relatively more activation in primary visual regions look for a prespecified visible speech target sequence (‘‘voo’’ (V1/V2), while visual movement areas (V5/MT+) were or ‘‘ahv’’) among other monosyllables. In one condition, the activated to a greater extent by moving faces. Cortical regions image sequence comprised a series of stilled key frames activated more by naturally moving speaking faces included showing apical gestures (e.g., separate frames for ‘‘v’’ and the auditory cortex (Brodmann’s Areas 41/42; lateral parts of ‘‘oo’’ [from the target] or ‘‘ee’’ and ‘‘m’’ [i.e., from Heschl’s gyrus) and the left STS and inferior frontal gyrus. -

In Brief in the Other Study, Jackson Et Al
RESEARCH HIGHLIGHTS CLEGR2+ neurons immediately before 43% of IPSPs driven by claustrum tones considerably reduced auditory activation were probably mediated population responses. by NPY neurons, whereas 35% IN briEF In the other study, Jackson et al. were mediated by FS neurons and used a retrograde virus approach to 22% by co-innervation by FS and SPATIAL NAVIGATION specifically target claustral neurons NPY neurons. Pharmacogenetic Planning a path projecting to the prefrontal cortex silencing of PV+ neurons (including (PFC) in mice (CL PFC neurons). FS neurons) or NPY neurons greatly Spatial navigation involves co-ordination between action → planning by the prefrontal cortex and spatial representation Optogenetic stimulation of these reduced the inhibitory responses of the environment in the hippocampus. In this study, when CL → PFC afferents led to a strong of pyramidal cells to claustral rats performed an alternating arm choice task in a T maze, the overall inhibition of pyramidal stimulation. Notably, when NPY coordination of the timing of spikes between neurons in neurons and inhibitory neurons in neurons were silenced, claustral the medial prefrontal cortex (mPFC), the thalamic nucleus the PFC. In acute slices, claustrum- stimulation even led to excitation reuniens (NR) and the hippocampal CA1 was found to increase; stimulated inhibitory responses of pyramidal cells, suggesting co-ordinated firing between supramammillary nucleus (SUM) and CA1 neurons also increased. Silencing of SUM neurons of prefrontal pyramidal cells were that claustrocortical excitation of decreased spike-time coordination in the mPFC-NR-CA1 blocked by glutamate receptor pyramidal cells is usually prevented circuit and impaired representations of the trajectory of antagonists, suggesting that the by NPY cell-mediated inhibition. -

Are the Neural Correlates of Consciousness in the Front Or in the Back of the Cerebral Cortex?
bioRxiv preprint doi: https://doi.org/10.1101/118273; this version posted March 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence Melanie Boly1,2*, Marcello Massimini3,4, Naotsugu Tsuchiya5, Bradley R. Postle2,6, Christof Koch7, Giulio Tononi2* 1 Department of Neurology, University of Wisconsin, Madison, WI, 53705 USA 2 Department of Psychiatry, University of Wisconsin, Madison, WI, 53719 USA 3 Department of Biomedical and Clinical Sciences ‘Luigi Sacco’, University of Milan, Milan, 20157, Italy 4 Instituto Di Ricovero e Cura a Carattere Scientifico, Fondazione Don Carlo Gnocchi, Milan, 20148, Italy 6 Department of Psychology, Monash University, Melbourne, 3168, Australia 6 Department of Psychology, University of Wisconsin, Madison, WI, 53705 USA 7 Allen Institute for Brain Science, Seattle, WA, 98109 USA Abbreviated title: Is consciousness in the front versus the back of corteX? Correspondence to: Melanie Boly: [email protected] or Giulio Tononi: [email protected]. Address: 6001 Research Park Boulevard, Madison WI 53719 Number of pages: 25 Number of figures: 5 Number of words for Abstract: 45; number of words for Main teXt: 3750 Conflicts of Interest: The authors declare no conflicts of interest. Acknowledgments: This work was supported by NIH/NINDS 1R03NS096379 (to M.B.), by the Tiny Blue Dot Foundation and the Distinguished Chair in Consciousness Science (University of Wisconsin) (to G.T.), and by the James S.