Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps Sinensis on the Treatment of Diabetic Nephropathy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dual Regulation of Planar Polarization by Secreted Wnts and Vangl2 in the Developing Mouse Cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A
© 2020. Published by The Company of Biologists Ltd | Development (2020) 147, dev191981. doi:10.1242/dev.191981 RESEARCH ARTICLE Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A. Quiruz1, Nicolas Grillet1 and Alan G. Cheng1,* ABSTRACT on the other. Flamingo (Fmi/Celsr1, Fmi/Celsr2 and Fmi/Celsr3) is Planar cell polarity (PCP) proteins localize asymmetrically to instruct present on both poles of the cell. Defective PCP signaling cell polarity within the tissue plane, with defects leading to deformities represented by a lack of polarized PCP components leads to of the limbs, neural tube and inner ear. Wnt proteins are evolutionarily congenital heart and tracheal abnormalities, skeletal dysplasia, conserved polarity cues, yet Wnt mutants display variable PCP neural tube defects as well as cochlear deformities (Butler and defects; thus, how Wnts regulate PCP remains unresolved. Here, we Wallingford, 2017; White et al., 2018). Despite their crucial roles, have used the developing cochlea as a model system to show that our understanding of upstream signals orchestrating PCP signaling secreted Wnts regulate PCP through polarizing a specific subset of is rather limited. PCP proteins. Conditional deletion of Wntless or porcupine, both of Wnt proteins have been implicated as upstream polarity cues for which are essential for secretion of Wnts, caused misrotated sensory PCP signaling. For example, limb morphogenesis in mice requires a cells and shortened cochlea – both hallmarks of PCP defects. gradient of Wnt5a, which has been reported to act as an instructive Wntless-deficient cochleae lacked the polarized PCP components cue to establish PCP (Gao et al., 2018, 2011). -

Association Between Chymase Gene Polymorphisms and Atrial Fibrillation
Zhou et al. BMC Cardiovascular Disorders (2019) 19:321 https://doi.org/10.1186/s12872-019-01300-7 RESEARCH ARTICLE Open Access Association between chymase gene polymorphisms and atrial fibrillation in Chinese Han population Dongchen Zhou, Yuewei Chen, Jiaxin Wu, Jiabo Shen, Yushan Shang, Liangrong Zheng and Xudong Xie* Abstract Background: Chymase is the major angiotensin II (Ang II)-forming enzyme in cardiovascular tissue, with an important role in atrial remodeling. This study aimed to examine the association between chymase 1 gene (CMA1) polymorphisms and atrial fibrillation (AF) in a Chinese Han population. Methods: This case-control study enrolled 126 patients with lone AF and 120 age- and sex-matched healthy controls, all from a Chinese Han population. Five CMA1 polymorphisms were genotyped. Results: The CMA1 polymorphism rs1800875 (G-1903A) was associated with AF. The frequency of the GG genotype was significantly higher in AF patients compared with controls (p = 0.009). Haplotype analysis further demonstrated an increased risk of AF associated with the rs1800875-G haplotype (Hap8 TGTTG, odds ratio (OR) = 1.668, 95% CI 1.132–2.458, p = 0.009), and a decreased risk for the rs1800875-A haplotype (Hap5 TATTG, OR = 0.178, 95% CI 0.042– 0.749, p = 0.008). Conclusions: CMA1 polymorphisms may be associated with AF, and the rs1800875 GG genotype might be a susceptibility factor for AF in the Chinese Han population. Keywords: Atrial fibrillation, CMA1, Chymase, Single nucleotide polymorphism, Angiotensin II Background ‘lone AF’, occur in the absence of identifiable underlying Atrial fibrillation (AF) is the most common type of sus- cardiovascular or other comorbid diseases [4]. -

Genome-Wide DNA Methylation Map of Human Neutrophils Reveals Widespread Inter-Individual Epigenetic Variation
www.nature.com/scientificreports OPEN Genome-wide DNA methylation map of human neutrophils reveals widespread inter-individual Received: 15 June 2015 Accepted: 29 October 2015 epigenetic variation Published: 27 November 2015 Aniruddha Chatterjee1,2, Peter A. Stockwell3, Euan J. Rodger1, Elizabeth J. Duncan2,4, Matthew F. Parry5, Robert J. Weeks1 & Ian M. Morison1,2 The extent of variation in DNA methylation patterns in healthy individuals is not yet well documented. Identification of inter-individual epigenetic variation is important for understanding phenotypic variation and disease susceptibility. Using neutrophils from a cohort of healthy individuals, we generated base-resolution DNA methylation maps to document inter-individual epigenetic variation. We identified 12851 autosomal inter-individual variably methylated fragments (iVMFs). Gene promoters were the least variable, whereas gene body and upstream regions showed higher variation in DNA methylation. The iVMFs were relatively enriched in repetitive elements compared to non-iVMFs, and were associated with genome regulation and chromatin function elements. Further, variably methylated genes were disproportionately associated with regulation of transcription, responsive function and signal transduction pathways. Transcriptome analysis indicates that iVMF methylation at differentially expressed exons has a positive correlation and local effect on the inclusion of that exon in the mRNA transcript. Methylation of DNA is a mechanism for regulating gene function in all vertebrates. It has a role in gene silencing, tissue differentiation, genomic imprinting, chromosome X inactivation, phenotypic plasticity, and disease susceptibility1,2. Aberrant DNA methylation has been implicated in the pathogenesis of sev- eral human diseases, especially cancer3–5. Variation in DNA methylation patterns in healthy individuals has been hypothesised to alter human phenotypes including susceptibility to common diseases6 and response to drug treatments7. -

The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS In
ISSN: 2378-3672 Asadi and Jamali. Int J Immunol Immunother 2019, 6:038 DOI: 10.23937/2378-3672/1410038 Volume 6 | Issue 1 International Journal of Open Access Immunology and Immunotherapy REVIEW ARTICLE The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS in Goltz Syndrome Shahin Asadi1* and Mahsa Jamali2 Check for updates Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran *Corresponding author: Shahin Asadi, Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran are usually formed around the nose, the lips, the anal Abstract holes and the genitals of women with Goltz syndrome. Goltz syndrome (focal skin hypoplasia) is a genetic disorder In addition, papillomas may be present in the throat, that primarily affects the skin, skeletal system, eyes and face. People with Goltz syndrome have birth defects. These especially in the esophagus or larynx, and can cause disorders include very thin skin veins (skin hypoplasia), pink swallowing, respiration or sleep poisoning. The papillo- yellow nodules, subcutaneous fat, lack of upper skin layers mas can be removed if necessary by surgery from the (aplasia cutis), small clusters of superficial skin vessels growing regions. People with Goltz syndrome may have (telangiectasia), and veins in dark skin Or bright. Goltz syndrome is caused by mutation genes PORCN, TWIST2, small and abnormal nails on the toes. In addition, hair in HCCS. the scalp of these people can be weak or fragile or not [2] (Figure 2). Keywords Goltz syndrome, PORCN, TWIST2, HCCS genes, Skin and Many people with Goltz syndrome also have hand Skeletal disorders and foot disorders, including abnormalities such as oligodactyly in fingers and toes, fingers and legs (cin- Generalizations of Goltz Syndrome dactyly) or ecd rhodactyly. -

CMA1 Rabbit Polyclonal Antibody – TA342809 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA342809 CMA1 Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: WB Recommended Dilution: WB Reactivity: Human Host: Rabbit Isotype: IgG Clonality: Polyclonal Immunogen: The immunogen for anti-CMA1 antibody: synthetic peptide directed towards the C terminal of human CMA1. Synthetic peptide located within the following region: EVKLRLMDPQACSHFRDFDHNLQLCVGNPRKTKSAFKGDSGGPLLCAGVA Formulation: Liquid. Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% sucrose. Note that this product is shipped as lyophilized powder to China customers. Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Predicted Protein Size: 27 kDa Gene Name: chymase 1 Database Link: NP_001827 Entrez Gene 1215 Human P23946 Background: CMA1 is a chymotryptic serine proteinase that belongs to the peptidase family S1. It is expressed in mast cells and thought to function in the degradation of the extracellular matrix, the regulation of submucosal gland secretion, and the generation of vasoactive peptides. In the heart and blood vessels, this protein, rather than angiotensin converting enzyme, is largely responsible for converting angiotensin I to the vasoactive peptide angiotensin II. Angiotensin II has been implicated in blood pressure control and in the pathogenesis of hypertension, cardiac hypertrophy, and heart failure. Thus, this gene product is a target for cardiovascular disease therapies. This product is to be used for laboratory only. Not for diagnostic or therapeutic use. -

A Uniform Human Wnt Expression Library Reveals a Shared Secretory Pathway and Unique Signaling Activities
Differentiation 84 (2012) 203–213 Contents lists available at SciVerse ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities Rani Najdi a, Kyle Proffitt b, Stephanie Sprowl a, Simran Kaur b, Jia Yu b, Tracy M. Covey b, David M. Virshup b,1, Marian L. Waterman a,n a Department of Microbiology and Molecular Genetics, University of California, Irvine, CA, USA b Program in Cancer and Stem Cell Biology, Duke-NUS Graduate Medical School, Singapore article info abstract Article history: Wnt ligands are secreted morphogens that control multiple developmental processes during embry- Received 24 April 2012 ogenesis and adult homeostasis. A diverse set of receptors and signals have been linked to individual Accepted 16 June 2012 Wnts, but the lack of tools for comparative analysis has limited the ability to determine which of these Available online 9 July 2012 signals are general for the entire Wnt family, and which define subsets of differently acting ligands. We Keywords: have created a versatile Gateway library of clones for all 19 human Wnts. An analysis comparing Wnt signaling epitope-tagged and untagged versions of each ligand shows that despite their similar expression at the b-catenin mRNA level, Wnts exhibit considerable variation in stability, processing and secretion. At least 14 out of Wnt modification and secretion the 19 Wnts activate b-catenin-dependent signaling, an activity that is cell type-dependent and tracks with the stabilization of b-catenin and LRP6 phosphorylation. We find that the core Wnt modification and secretion proteins Porcupine (PORCN) and Wntless (WLS) are essential for all Wnts to signal through b-catenin-dependent and independent pathways. -
Primepcr™Assay Validation Report
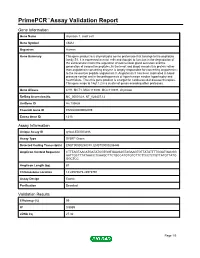
PrimePCR™Assay Validation Report Gene Information Gene Name chymase 1, mast cell Gene Symbol CMA1 Organism Human Gene Summary This gene product is a chymotryptic serine proteinase that belongs to the peptidase family S1. It is expressed in mast cells and thought to function in the degradation of the extracellular matrix the regulation of submucosal gland secretion and the generation of vasoactive peptides. In the heart and blood vessels this protein rather than angiotensin converting enzyme is largely responsible for converting angiotensin I to the vasoactive peptide angiotensin II. Angiotensin II has been implicated in blood pressure control and in the pathogenesis of hypertension cardiac hypertrophy and heart failure. Thus this gene product is a target for cardiovascular disease therapies. This gene maps to 14q11.2 in a cluster of genes encoding other proteases. Gene Aliases CYH, MCT1, MGC119890, MGC119891, chymase RefSeq Accession No. NC_000014.8, NT_026437.12 UniGene ID Hs.135626 Ensembl Gene ID ENSG00000092009 Entrez Gene ID 1215 Assay Information Unique Assay ID qHsaCED0003486 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000250378, ENST00000206446 Amplicon Context Sequence CTTTAGTAACATGATATCGTGGTGAAGAGTAGAAGTGTTATATTTTGGATGACGG AATTGCTTTATAACCTCAAGCTTCTGCCATGTGTCTTCTTCCTCTGTTATGTTATG GGCTCC Amplicon Length (bp) 87 Chromosome Location 14:24975675-24975791 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 99 R2 0.9989 cDNA Cq 27.32 Page 1/5 PrimePCR™Assay Validation Report cDNA Tm (Celsius) 78 -

Gene Expression Profiling of Lymph Node Sub-Capsular Sinus Macrophages in Cancer
ORIGINAL RESEARCH published: 08 June 2021 doi: 10.3389/fimmu.2021.672123 Gene Expression Profiling of Lymph Node Sub-Capsular Sinus Macrophages in Cancer † † Danilo Pellin 1 , Natalie Claudio 2,3 , Zihan Guo 2,4, Tahereh Ziglari 2 and Ferdinando Pucci 2,3* 1 Gene Therapy Program, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Harvard Medical School, Boston, MA, United States, 2 Department of Otolaryngology – Head and Neck Surgery, Oregon Health and Science University, Portland, OR, United States, 3 Department of Cell, Developmental & Cancer Biology, Oregon Health and Science University, Portland, OR, United States, 4 Program in Cancer Biology, Oregon Health and Science University, Portland, OR, United States Lymph nodes are key lymphoid organs collecting lymph fluid and migratory cells from the tissue area they survey. When cancerous cells arise within a tissue, the sentinel lymph node is the first immunological organ to mount an immune response. Sub-capsular sinus Edited by: Karine Rachel Prudent Breckpot, macrophages (SSMs) are specialized macrophages residing in the lymph nodes that play Vrije University Brussel, Belgium important roles as gatekeepers against particulate antigenic material. In the context of Reviewed by: cancer, SSMs capture tumor-derived extracellular vesicles (tEVs), a form of particulate Ioannis S. Pateras, National and Kapodistrian University of antigen released in high amounts by tumor cells. We and others have recently Athens, Greece demonstrated that SSMs possess anti-tumor activity because in their absence tumors Antonio Giovanni Solimando, progress faster. A comprehensive profiling of SSMs represents an important first step to University of Bari Aldo Moro, Italy Rohit Singh, identify the cellular and molecular mechanisms responsible for SSM anti-tumor activity. -

PRCP) As a New Target for Obesity Treatment
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Prolylcarboxypeptidase (PRCP) as a new target for obesity treatment B Shariat-Madar1 Abstract: Recently, we serendipitously discovered that mice with the deficiency of the enzyme D Kolte2 prolylcarboxypeptidase (PRCP) have elevated α-melanocyte-stimulating hormone (α-MSH) A Verlangieri2 levels which lead to decreased food intake and weight loss. This suggests that PRCP is an Z Shariat-Madar2 endogenous inactivator of α-MSH and an appetite stimulant. Since a modest weight loss can have the most profound influence on reducing cardiovascular risk factors, the inhibitors of PRCP 1College of Literature, Science, and would be emerging as a possible alternative for pharmacotherapy in high-risk patients with the Arts, University of Michigan, Ann Arbor MI, USA; 2School obesity and obesity-related disorders. The discovery of a new biological activity of PRCP in the of Pharmacy, Department PRCP-deficient mice and studies of α-MSH function indicate the importance and complexity of Pharmacology, University of Mississippi, University, of the hypothalamic pro-opiomelanocortin (POMC) system in altering food intake. Identifying MS, USA a role for PRCP in regulating α-MSH in the brain may be a critical step in enhancing our understanding of how the brain controls food intake and body weight. In light of recent findings, the potential role of PRCP in regulating fuel homeostasis is critically evaluated. Further studies of the role of PRCP in obesity are much needed. Keywords: prolylcarboxypeptidase, melanocyte-stimulating harmone, appetite, weight loss, cardiovascular risk, obesity Introduction The hypothalamus is often times a target for newer potential obesity treatments due to its crucial role in food intake and metabolism. -

Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis
Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis by Valbona Luga A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, Department of Molecular Genetics, University of Toronto © Copyright by Valbona Luga, 2013 Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis Valbona Luga Doctor of Philosophy Department of Molecular Genetics University of Toronto 2013 Abstract The tumour-associated stroma, consisting of fibroblasts, inflammatory cells, vasculature and extracellular matrix proteins, plays a critical role in tumour growth, but how it regulates cancer cell migration and metastasis is poorly understood. The Wnt-planar cell polarity (PCP) pathway regulates convergent extension movements in vertebrate development. However, it is unclear whether this pathway also functions in cancer cell migration. In addition, the factors that mobilize long-range signalling of Wnt morphogens, which are tightly associated with the plasma membrane, have yet to be completely characterized. Here, I show that fibroblasts secrete membrane microvesicles of endocytic origin, termed exosomes, which promote tumour cell protrusive activity, motility and metastasis via the exosome component Cd81. In addition, I demonstrate that fibroblast exosomes activate autocrine Wnt-PCP signalling in breast cancer cells as detected by the association of Wnt with Fzd receptors and the asymmetric distribution of Fzd-Dvl and Vangl-Pk complexes in exosome-stimulated cancer cell protrusive structures. Moreover, I show that Pk expression in breast cancer cells is essential for fibroblast-stimulated cancer cell metastasis. Lastly, I reveal that trafficking in cancer cells promotes tethering of autocrine Wnt11 to fibroblast exosomes. These studies further our understanding of the role of ii the tumour-associated stroma in cancer metastasis and bring us closer to a more targeted approach for the treatment of cancer spread. -
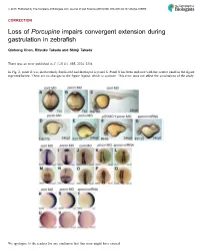
Porcupine Impairs Convergent Extension During Gastrulation in Zebrafish
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 000, 000–000 doi:10.1242/jcs.168989 CORRECTION Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen, Ritsuko Takada and Shinji Takada There was an error published in J. Cell Sci. 125, 2224–2234. In Fig. 2, panel R was inadvertently duplicated and displayed as panel S. Panel S has been replaced with the correct panel in the figure reprinted below. There are no changes to the figure legend, which is accurate. This error does not affect the conclusions of the study. We apologise to the readers for any confusion that this error might have caused. 1 Journal of Cell Science JCS168989.3d 22/1/15 10:57:22 The Charlesworth Group, Wakefield +44(0)1924 369598 - Rev 9.0.225/W (Oct 13 2006) 2224 Research Article Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen1, Ritsuko Takada1 and Shinji Takada1,2,* 1Okazaki Institute for Integrative Bioscience and National Institute for Basic Biology, National Institutes of Natural Sciences, Okazaki, Aichi 444-8787, Japan 2Department of Molecular Biomechanics, The Graduate University for Advanced Studies (SOKENDAI), Okazaki, Aichi 444-8787, Japan *Author for correspondence ([email protected]) Accepted 9 January 2012 Journal of Cell Science 125, 2224–2234 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098368 Summary Porcupine (Porcn), an O-acyltransferase located in the endoplasmic reticulum (ER), is required for lipidation of Wnt proteins to enable their trafficking from the ER in mammalian cell culture. -

Refined Genetic Mapping of Autosomal Recessive Chronic Distal Spinal Muscular Atrophy to Chromosome 11Q13.3 and Evidence of Link
European Journal of Human Genetics (2004) 12, 483–488 & 2004 Nature Publishing Group All rights reserved 1018-4813/04 $30.00 www.nature.com/ejhg ARTICLE Refined genetic mapping of autosomal recessive chronic distal spinal muscular atrophy to chromosome 11q13.3 and evidence of linkage disequilibrium in European families Louis Viollet*,1, Mohammed Zarhrate1, Isabelle Maystadt1, Brigitte Estournet-Mathiaut2, Annie Barois2, Isabelle Desguerre3, Miche`le Mayer4, Brigitte Chabrol5, Bruno LeHeup6, Veronica Cusin7, Thierry Billette de Villemeur8, Dominique Bonneau9, Pascale Saugier-Veber10, Anne Touzery-de Villepin11, Anne Delaubier12, Jocelyne Kaplan1, Marc Jeanpierre13, Joshue´ Feingold1 and Arnold Munnich1 1Unite´ de Recherches sur les Handicaps Ge´ne´tiques de l’Enfant, INSERM U393. Hoˆpital Necker Enfants Malades, 149 rue de Se`vres, 75743 Paris Cedex 15, France; 2Service de Neurope´diatrie, Re´animation et Re´e´ducation Neuro-respiratoire, Hoˆpital Raymond Poincare´, 92380 Garches, France; 3Service de Neurope´diatrie, Hoˆpital Necker Enfants Malades, 149 rue de Se`vres, 75743 Paris Cedex 15, France; 4Service de Neurope´diatrie, Hoˆpital Saint Vincent de Paul, 82 boulevard Denfert Rochereau, 75674 Paris Cedex 14, France; 5Service de Neurope´diatrie, Hoˆpital Timone Enfants, 264 rue Saint Pierre 13385 Marseille Cedex, France; 6Secteur de De´veloppement et Ge´ne´tique, CHR de Nancy, Hoˆpitaux de Brabois, Rue du Morvan, 54511 Vandoeuvre Cedex, France; 7Service de Ge´ne´tique de Dijon, Hoˆpital d’Enfants, 2 blvd du Mare´chal de Lattre de Tassigny,