A Uniform Human Wnt Expression Library Reveals a Shared Secretory Pathway and Unique Signaling Activities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dual Regulation of Planar Polarization by Secreted Wnts and Vangl2 in the Developing Mouse Cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A
© 2020. Published by The Company of Biologists Ltd | Development (2020) 147, dev191981. doi:10.1242/dev.191981 RESEARCH ARTICLE Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A. Quiruz1, Nicolas Grillet1 and Alan G. Cheng1,* ABSTRACT on the other. Flamingo (Fmi/Celsr1, Fmi/Celsr2 and Fmi/Celsr3) is Planar cell polarity (PCP) proteins localize asymmetrically to instruct present on both poles of the cell. Defective PCP signaling cell polarity within the tissue plane, with defects leading to deformities represented by a lack of polarized PCP components leads to of the limbs, neural tube and inner ear. Wnt proteins are evolutionarily congenital heart and tracheal abnormalities, skeletal dysplasia, conserved polarity cues, yet Wnt mutants display variable PCP neural tube defects as well as cochlear deformities (Butler and defects; thus, how Wnts regulate PCP remains unresolved. Here, we Wallingford, 2017; White et al., 2018). Despite their crucial roles, have used the developing cochlea as a model system to show that our understanding of upstream signals orchestrating PCP signaling secreted Wnts regulate PCP through polarizing a specific subset of is rather limited. PCP proteins. Conditional deletion of Wntless or porcupine, both of Wnt proteins have been implicated as upstream polarity cues for which are essential for secretion of Wnts, caused misrotated sensory PCP signaling. For example, limb morphogenesis in mice requires a cells and shortened cochlea – both hallmarks of PCP defects. gradient of Wnt5a, which has been reported to act as an instructive Wntless-deficient cochleae lacked the polarized PCP components cue to establish PCP (Gao et al., 2018, 2011). -

The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS In
ISSN: 2378-3672 Asadi and Jamali. Int J Immunol Immunother 2019, 6:038 DOI: 10.23937/2378-3672/1410038 Volume 6 | Issue 1 International Journal of Open Access Immunology and Immunotherapy REVIEW ARTICLE The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS in Goltz Syndrome Shahin Asadi1* and Mahsa Jamali2 Check for updates Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran *Corresponding author: Shahin Asadi, Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran are usually formed around the nose, the lips, the anal Abstract holes and the genitals of women with Goltz syndrome. Goltz syndrome (focal skin hypoplasia) is a genetic disorder In addition, papillomas may be present in the throat, that primarily affects the skin, skeletal system, eyes and face. People with Goltz syndrome have birth defects. These especially in the esophagus or larynx, and can cause disorders include very thin skin veins (skin hypoplasia), pink swallowing, respiration or sleep poisoning. The papillo- yellow nodules, subcutaneous fat, lack of upper skin layers mas can be removed if necessary by surgery from the (aplasia cutis), small clusters of superficial skin vessels growing regions. People with Goltz syndrome may have (telangiectasia), and veins in dark skin Or bright. Goltz syndrome is caused by mutation genes PORCN, TWIST2, small and abnormal nails on the toes. In addition, hair in HCCS. the scalp of these people can be weak or fragile or not [2] (Figure 2). Keywords Goltz syndrome, PORCN, TWIST2, HCCS genes, Skin and Many people with Goltz syndrome also have hand Skeletal disorders and foot disorders, including abnormalities such as oligodactyly in fingers and toes, fingers and legs (cin- Generalizations of Goltz Syndrome dactyly) or ecd rhodactyly. -

Deregulated Wnt/Β-Catenin Program in High-Risk Neuroblastomas Without
Oncogene (2008) 27, 1478–1488 & 2008 Nature Publishing Group All rights reserved 0950-9232/08 $30.00 www.nature.com/onc ONCOGENOMICS Deregulated Wnt/b-catenin program in high-risk neuroblastomas without MYCN amplification X Liu1, P Mazanek1, V Dam1, Q Wang1, H Zhao2, R Guo2, J Jagannathan1, A Cnaan2, JM Maris1,3 and MD Hogarty1,3 1Division of Oncology, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA; 2Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA and 3Department of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA Neuroblastoma (NB) is a frequently lethal tumor of Introduction childhood. MYCN amplification accounts for the aggres- sive phenotype in a subset while the majority have no Neuroblastoma (NB) is a childhood embryonal malig- consistently identified molecular aberration but frequently nancy arising in the peripheral sympathetic nervous express MYC at high levels. We hypothesized that acti- system. Half of all children with NB present with features vated Wnt/b-catenin (CTNNB1) signaling might account that define their tumorsashigh riskwith poor overall for this as MYC is a b-catenin transcriptional target and survival despite intensive therapy (Matthay et al., 1999). multiple embryonal and neural crest malignancies have A subset of these tumors are characterized by high-level oncogenic alterations in this pathway. NB cell lines without genomic amplification of the MYCN proto-oncogene MYCN amplification express higher levels of MYC and (Matthay et al., 1999) but the remainder have no b-catenin (with aberrant nuclear localization) than MYCN- consistently identified aberration to account for their amplified cell lines. -

Towards an Integrated View of Wnt Signaling in Development Renée Van Amerongen and Roel Nusse*
HYPOTHESIS 3205 Development 136, 3205-3214 (2009) doi:10.1242/dev.033910 Towards an integrated view of Wnt signaling in development Renée van Amerongen and Roel Nusse* Wnt signaling is crucial for embryonic development in all animal Notably, components at virtually every level of the Wnt signal species studied to date. The interaction between Wnt proteins transduction cascade have been shown to affect both β-catenin- and cell surface receptors can result in a variety of intracellular dependent and -independent responses, depending on the cellular responses. A key remaining question is how these specific context. As we discuss below, this holds true for the Wnt proteins responses take shape in the context of a complex, multicellular themselves, as well as for their receptors and some intracellular organism. Recent studies suggest that we have to revise some of messengers. Rather than concluding that these proteins are shared our most basic ideas about Wnt signal transduction. Rather than between pathways, we instead propose that it is the total net thinking about Wnt signaling in terms of distinct, linear, cellular balance of signals that ultimately determines the response of the signaling pathways, we propose a novel view that considers the receiving cell. In the context of an intact and developing integration of multiple, often simultaneous, inputs at the level organism, cells receive multiple, dynamic, often simultaneous and of both Wnt-receptor binding and the downstream, sometimes even conflicting inputs, all of which are integrated to intracellular response. elicit the appropriate cell behavior in response. As such, the different signaling pathways might thus be more intimately Introduction intertwined than previously envisioned. -

Hippocampus Formation: an Intriguing Collaboration Henk Roelink
bb10g06.qxd 04/03/2000 01:10 Page R279 Dispatch R279 Hippocampus formation: An intriguing collaboration Henk Roelink Recent genetic studies have shown that the signalling temporal lobes are formed. In an adult rodent, the hip- factor Wnt3a is required for formation of the pocampus is consequently still found close to the dorsal hippocampus; the developmental consequences of Wnt midline where it is initially formed (Figure 1). signalling in the hippocampus are mediated by multiple HMG-box transcription factors, with LEF-1 being It is clear that Wnt family members are required for the required just for formation of the dentate gyrus. development of many embryonic structures, functions mediated by their effects on fundamental processes such Address: Department of Biological Structure and Center for Developmental Biology, University of Washington, Box 357420, as cell proliferation, differentiation, survival or mainte- Seattle, Washington 98117-7420, USA. nance. Obtaining a clear picture of how each Wnt acts is E-mail: [email protected] complicated by the relatively large size of the family, which has at least 18 members in amniotes [3]. Analyses of Current Biology 2000, 10:R279–R281 mice lacking one or several Wnt genes have revealed some 0960-9822/00/$ – see front matter remarkable phenotypes, often characterized by failure of © 2000 Elsevier Science Ltd. All rights reserved. induction of very specific anatomical structures. Wnt3A is no exception, and although several papers have been pub- Inductive interactions are fundamental to the formation of lished already on the more obvious defects of Wnt3a all brain structures. and signalling by molecules of the knockout mice, a recent paper from McMahon and col- Wingless/Wnt family is known to play a role in many of leagues [1] has reported a very interesting defect in the them. -

Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps Sinensis on the Treatment of Diabetic Nephropathy
Hindawi Journal of Diabetes Research Volume 2021, Article ID 8891093, 12 pages https://doi.org/10.1155/2021/8891093 Research Article Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps sinensis on the Treatment of Diabetic Nephropathy Yan Li,1 Lei Wang,2 Bojun Xu,1 Liangbin Zhao,1 Li Li,1 Keyang Xu,3 Anqi Tang,1 Shasha Zhou,1 Lu Song,1 Xiao Zhang,1 and Huakui Zhan 1 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, 610072 Sichuan, China 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China 3Zhejiang Chinese Medical University, Hangzhou, 310053 Zhejiang, China Correspondence should be addressed to Huakui Zhan; [email protected] Received 27 August 2020; Revised 17 January 2021; Accepted 24 January 2021; Published 8 February 2021 Academic Editor: Michaelangela Barbieri Copyright © 2021 Yan Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus and is a major cause of end- stage kidney disease. Cordyceps sinensis (Cordyceps, Dong Chong Xia Cao) is a widely applied ingredient for treating patients with DN in China, while the molecular mechanisms remain unclear. This study is aimed at revealing the therapeutic mechanisms of Cordyceps in DN by undertaking a network pharmacology analysis. Materials and Methods. In this study, active ingredients and associated target proteins of Cordyceps sinensis were obtained via Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Swiss Target Prediction platform, then reconfirmed by using PubChem databases. -

Wnt3a: Functions and Implications in Cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T
He et al. Chin J Cancer (2015) 34:50 DOI 10.1186/s40880-015-0052-4 REVIEW Open Access Wnt3a: functions and implications in cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T. Keller3, Chao‑Nan Qian4* and Jian Zhang1,2,3* Abstract Wnt3a, one of Wnt family members, plays key roles in regulating pleiotropic cellular functions, including self-renewal, proliferation, differentiation, and motility. Accumulating evidence has suggested that Wnt3a promotes or suppresses tumor progression via the canonical Wnt signaling pathway depending on cancer type. In addition, the roles of Wnt3a signaling can be inhibited by multiple proteins or chemicals. Herein, we summarize the latest findings on Wnt3a as an important therapeutic target in cancer. Keywords: Wnt3a, The Wnt signaling pathway, Cancer Background: the Wnt signaling pathway cell polarity pathway (Wnt-PCP pathway) and the Wnt- The Wnt gene was first identified in 1982 and is named calcium pathway (Wnt-Ca2+ pathway) [5]. According to the Int gene in mice [1]. A subsequent study reported the characteristics of its functions, the Wnt family can that the Int gene and wingless gene in Drosophila were also be divided into two categories: Wnt1 and Wnt5a. homologous genes [2]. Therefore, both genes were rec- The Wnt1 category includes Wnt1, Wnt2, Wnt2b, Wnt3, ognized as the Wnt gene [3]. The Wnt family comprises Wnt3a, Wnt7a, Wnt8, Wnt8b, and Wnt10a, which are 19 human proteins, including Wnt1, Wnt2, Wnt2b involved in the canonical signaling pathway, whereas (Wnt13), Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt4, Wnt5a, and Wnt11 belong to the Wnt5a category Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a (Wnt14), Wnt9b and activate the noncanonical signaling pathway. -

Adenomatous Polyposis Coli Is Required for Early Events in the Normal Growth and Differentiation of the Developing Cerebral Cortex
Edinburgh Research Explorer Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex Citation for published version: Ivaniutsin, U, Chen, Y, Mason, JO, Price, D & Pratt, T 2009, 'Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex', Neural Development, vol. 4, no. n/a, 3, pp. -. https://doi.org/10.1186/1749-8104-4-3 Digital Object Identifier (DOI): 10.1186/1749-8104-4-3 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Neural Development Publisher Rights Statement: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing -

Analysis of the Canonical WNT Pathway Simona Giunta Department of Biological Sciences, Brunel University, Uxbridge, Middlesex, UK
05-giunta 25-02-2010 15:21 Pagina 187 ACTA BIOMED 2009; 80: 187-199 © Mattioli 1885 R EVIEW A gust of WNT: analysis of the canonical WNT pathway Simona Giunta Department of Biological Sciences, Brunel University, Uxbridge, Middlesex, UK Abstract. The Wnt pathway is a signal-transduction cascade that mediates communication between cells; the Wnt pathway is involved in key steps during embryological development and in the maintenance of adult tissue homeostasis. Mutational dysregulation of Wnt cascade components has been observed in diverse hu- man pathological conditions and in oncogenic transformations. For these reasons, the Wnt signalling path- way has acquired growing interest in scientific and medical research over recent years. This review outlines the biochemical and functional features of the Wnt cascade with particular emphasis on a detailed function- al analysis of all key players. In this instance, the regulations of the pathway have also been covered, empha- sizing novelty in this regard. Furthermore, past and present studies on Wnt have been included, as well as a prediction of scientific progress, which may be made in this rapidly evolving field, in the near future; the re- view also embraces considerations on how further understanding of the Wnt pathway will provide important insight into managing human diseases. (www.actabiomedica.it) Key words: Wnt canonical pathway, b-catenin, signal transduction, haematopoietic stem cells, carcinogenesis Introduction molecules which regulate the main steps of embryoge- nesis. Multicellular organisms necessitate a communi- The Wnt family of signalling proteins has been cation system to grow and function; in complex mul- found to be involved in embryonic patterning, in the ticellular organisms, like humans, cell-to-cell commu- homeostasis of adult tissue self-renewal and in the nication becomes the basis of life. -

Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis
Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis by Valbona Luga A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, Department of Molecular Genetics, University of Toronto © Copyright by Valbona Luga, 2013 Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis Valbona Luga Doctor of Philosophy Department of Molecular Genetics University of Toronto 2013 Abstract The tumour-associated stroma, consisting of fibroblasts, inflammatory cells, vasculature and extracellular matrix proteins, plays a critical role in tumour growth, but how it regulates cancer cell migration and metastasis is poorly understood. The Wnt-planar cell polarity (PCP) pathway regulates convergent extension movements in vertebrate development. However, it is unclear whether this pathway also functions in cancer cell migration. In addition, the factors that mobilize long-range signalling of Wnt morphogens, which are tightly associated with the plasma membrane, have yet to be completely characterized. Here, I show that fibroblasts secrete membrane microvesicles of endocytic origin, termed exosomes, which promote tumour cell protrusive activity, motility and metastasis via the exosome component Cd81. In addition, I demonstrate that fibroblast exosomes activate autocrine Wnt-PCP signalling in breast cancer cells as detected by the association of Wnt with Fzd receptors and the asymmetric distribution of Fzd-Dvl and Vangl-Pk complexes in exosome-stimulated cancer cell protrusive structures. Moreover, I show that Pk expression in breast cancer cells is essential for fibroblast-stimulated cancer cell metastasis. Lastly, I reveal that trafficking in cancer cells promotes tethering of autocrine Wnt11 to fibroblast exosomes. These studies further our understanding of the role of ii the tumour-associated stroma in cancer metastasis and bring us closer to a more targeted approach for the treatment of cancer spread. -
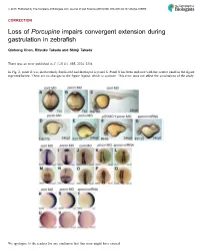
Porcupine Impairs Convergent Extension During Gastrulation in Zebrafish
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 000, 000–000 doi:10.1242/jcs.168989 CORRECTION Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen, Ritsuko Takada and Shinji Takada There was an error published in J. Cell Sci. 125, 2224–2234. In Fig. 2, panel R was inadvertently duplicated and displayed as panel S. Panel S has been replaced with the correct panel in the figure reprinted below. There are no changes to the figure legend, which is accurate. This error does not affect the conclusions of the study. We apologise to the readers for any confusion that this error might have caused. 1 Journal of Cell Science JCS168989.3d 22/1/15 10:57:22 The Charlesworth Group, Wakefield +44(0)1924 369598 - Rev 9.0.225/W (Oct 13 2006) 2224 Research Article Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen1, Ritsuko Takada1 and Shinji Takada1,2,* 1Okazaki Institute for Integrative Bioscience and National Institute for Basic Biology, National Institutes of Natural Sciences, Okazaki, Aichi 444-8787, Japan 2Department of Molecular Biomechanics, The Graduate University for Advanced Studies (SOKENDAI), Okazaki, Aichi 444-8787, Japan *Author for correspondence ([email protected]) Accepted 9 January 2012 Journal of Cell Science 125, 2224–2234 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098368 Summary Porcupine (Porcn), an O-acyltransferase located in the endoplasmic reticulum (ER), is required for lipidation of Wnt proteins to enable their trafficking from the ER in mammalian cell culture. -

Microrna-124-3P Suppresses Mouse Lip Mesenchymal Cell Proliferation
Suzuki et al. BMC Genomics (2019) 20:852 https://doi.org/10.1186/s12864-019-6238-4 RESEARCH ARTICLE Open Access MicroRNA-124-3p suppresses mouse lip mesenchymal cell proliferation through the regulation of genes associated with cleft lip in the mouse Akiko Suzuki1,2, Hiroki Yoshioka1,2, Dima Summakia1, Neha G. Desai1,3, Goo Jun3,4, Peilin Jia5, David S. Loose4,6, Kenichi Ogata1,2, Mona V. Gajera1,3, Zhongming Zhao3,4,5 and Junichi Iwata1,2,4* Abstract Background: Cleft lip (CL), one of the most common congenital birth defects, shows considerable geographic and ethnic variation, with contribution of both genetic and environmental factors. Mouse genetic studies have identified several CL-associated genes. However, it remains elusive how these CL-associated genes are regulated and involved in CL. Environmental factors may regulate these genes at the post-transcriptional level through the regulation of non-coding microRNAs (miRNAs). In this study, we sought to identify miRNAs associated with CL in mice. Results: Through a systematic literature review and a Mouse Genome Informatics (MGI) database search, we identified 55 genes that were associated with CL in mice. Subsequent bioinformatic analysis of these genes predicted that a total of 33 miRNAs target multiple CL-associated genes, with 20 CL-associated genes being potentially regulated by multiple miRNAs. To experimentally validate miRNA function in cell proliferation, we conducted cell proliferation/viability assays for the selected five candidate miRNAs (miR-124-3p, let-7a-5p, let-7b-5p, let-7c-5p, and let-7d-5p). Overexpression of miR-124-3p, but not of the others, inhibited cell proliferation through suppression of CL-associated genes in cultured mouse embryonic lip mesenchymal cells (MELM cells) isolated from the developing mouse lip region.