USDA Database for the Isoflavone Content of Selected Foods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Content and Composition of Isoflavonoids in Mature Or Immature
394 Journal of Health Science, 47(4) 394–406 (2001) Content and Composition of tivities1) and reported to be protective against can- cer, cardiovascular diseases and osteoporosis.3–9) Isoflavonoids in Mature or Much research has been reported about the content Immature Beans and Bean of isoflavonoids in soybeans and soybean-derived processed foods.10–23) In contrast, there are few re- Sprouts Consumed in Japan ports about the isoflavonoid content in beans other than soybeans.11,12,18,23) Yumiko Nakamura,* Akiko Kaihara, Japanese people are reported to ingest Kimihiko Yoshii, Yukari Tsumura, isoflavonoids mainly through the consumption of Susumu Ishimitsu, and Yasuhide Tonogai soybeans and its derived processed foods.20) Re- cently, we estimated that the Japanese daily intake Division of Food Chemistry, National Institute of Health Sci- of isoflavonoids from soybeans and soybean-based ences, Osaka Branch, 1–1–43 Hoenzaka, Chuo-ku, Osaka 540– processed foods is 27.80 mg per day (daidzein 0006, Japan (Received January 9, 2001; Accepted April 6, 2001) 12.02 mg, glycitein 2.30 mg and genistein 13.48 mg).24) However, isoflavonoid intake from the The content of 9 types of isoflavonoids (daidzein, consumption of immature beans, sprouts and beans glycitein, genistein, formononetin, biochanin A, other than soybeans has not been elucidated. Here coumestrol, daidzin, glycitin and genistin) in 34 do- we have measured the content of isoflavonoids in mestic or imported raw beans including soybeans, 7 mature and immature beans and bean sprouts con- immature beans and 5 bean sprouts consumed in Ja- sumed in Japan, and have compared the content and pan were systematically analyzed. -

Interpreting Sources and Endocrine Active Components of Trace Organic Contaminant Mixtures in Minnesota Lakes
INTERPRETING SOURCES AND ENDOCRINE ACTIVE COMPONENTS OF TRACE ORGANIC CONTAMINANT MIXTURES IN MINNESOTA LAKES by Meaghan E. Guyader © Copyright by Meaghan E. Guyader, 2018 All Rights Reserved A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Civil and Environmental Engineering). Golden, Colorado Date _____________________________ Signed: _____________________________ Meaghan E. Guyader Signed: _____________________________ Dr. Christopher P. Higgins Thesis Advisor Golden, Colorado Date _____________________________ Signed: _____________________________ Dr. Terri S. Hogue Professor and Department Head Department of Civil and Environmental Engineering ii ABSTRACT On-site wastewater treatment systems (OWTSs) are a suspected source of widespread trace organic contaminant (TOrC) occurrence in Minnesota lakes. TOrCs are a diverse set of synthetic and natural chemicals regularly used as cleaning agents, personal care products, medicinal substances, herbicides and pesticides, and foods or flavorings. Wastewater streams are known to concentrate TOrC discharges to the environment, particularly accumulating these chemicals at outfalls from centralized wastewater treatment plants. Fish inhabiting these effluent dominated environments are also known to display intersex qualities. Concurrent evidence of this phenomenon, known as endocrine disruption, in Minnesota lake fish drives hypotheses that OWTSs, the primary form of wastewater treatment in shoreline residences, may contribute to TOrC occurrence and the endocrine activity in these water bodies. The causative agents specific to fish in this region remain poorly understood. The objective of this dissertation was to investigate OWTSs as sources of TOrCs in Minnesota lakes, and TOrCs as potential causative agents for endocrine disruption in resident fish. -

Nutraceuticals Brian Lockwood
CjigVXZji^XVah HZXdcYZY^i^dc 7g^VcAdX`lddY 00 Prelim 2/3/07 18:51 Page i Nutraceuticals 00 Prelim 2/3/07 18:51 Page ii 00 Prelim 2/3/07 18:51 Page iii Nutraceuticals A guide for healthcare professionals Second edition Brian Lockwood BPharm, PhD, MRPharmS Senior Lecturer in Pharmacy, School of Pharmacy and Pharmaceutical Sciences, University of Manchester, Manchester, UK London • Chicago 00 Prelim 2/3/07 18:51 Page iv Published by the Pharmaceutical Press An imprint of RPS Publishing 1 Lambeth High Street, London SE1 7JN, UK 100 South Atkinson Road, Suite 200, Grayslake, IL 60030–7820, USA © Pharmaceutical Press 2007 is a trade mark of RPS Publishing RPS Publishing is the publishing organisation of the Royal Pharmaceutical Society of Great Britain First edition published 2002 Second edition published 2007 Typeset by Type Study, Scarborough, North Yorkshire Printed in Great Britain by TJ International, Padstow, Cornwall ISBN 978 0 85369 659 9 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior written permission of the copyright holder. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. The right of Brian Lockwood to be identified as the author of this work has been asserted by him in accordance with sections 77 and 78 and subject to section 79(6) of the Copyright, Designs and Patents Act, 1988. -

1 1 2 3 4 5 6 7 Bioactive Phenolic Compounds of Soybean
* Manuscript Click here to view linked References 1 Bioactive phenolic compounds of soybean ( Glycine max cv. Merit): modifications by 2 different microbial fermentations 3 4 5 M. Dueñas 2; T. Hernández 1; S. Robredo 1; *I. Estrella 1; R. Muñoz 1 6 7 1Instituto de Fermentaciones Industriales. C.S.I.C. Juan de la Cierva 3, 28006-Madrid. Spain. Fax: 34-915644853. 8 [email protected] 9 2Grupo de Investigación en Polifenoles, Unidad de Nutrición y Bromatología, Facultad de Farmacia, Universidad de 10 Salamanca. Campus Miguel de Unamuno. 37007 Salamanca. Spain. 11 12 *Corresponding author 13 14 Running title: Changes in phenolic compounds of soybeans by microbial fermentations 15 16 17 Abstract 18 Phenolic compounds of soybeans ( Glycine max ) are partially responsible of their beneficial 19 health effects. The most abundant phenolics in soybeans are isoflavones, mainly daidzein, 20 glycitein, and genistein and several derivatives of them. Other phenolic compounds are also 21 present but they have been minimally studied. In this work, the soybean seeds were fermented by 22 the microbiota present on the seeds or by inoculation with Aspergillus oryzae, Rhizopus oryzae or 23 Bacillus subtilis . The effect of microbial fermentation on the phenolic composition of soybeans 24 was studied. All the assayed microorganisms brought out variations in phenolics. An increase in 25 the concentration of phenolic acids was observed. Flavonoids were differently metabolized 26 depending on the microorganism used. In all samples the most important changes were produced 27 in the isoflavones content, observing an increase on the aglycones, glycitein, daidzein and 28 genistein. 29 30 Key words : soybean, fermentation, phenolic compounds, isoflavones 31 1 32 1. -

Memorandum Date: June 6, 2014
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Memorandum Date: June 6, 2014 From: Bisphenol A (BPA) Joint Emerging Science Working Group Smita Baid Abraham, M.D. ∂, M. M. Cecilia Aguila, D.V.M. ⌂, Steven Anderson, Ph.D., M.P.P.€* , Jason Aungst, Ph.D.£*, John Bowyer, Ph.D. ∞, Ronald P Brown, M.S., D.A.B.T.¥, Karim A. Calis, Pharm.D., M.P.H. ∂, Luísa Camacho, Ph.D. ∞, Jamie Carpenter, Ph.D.¥, William H. Chong, M.D. ∂, Chrissy J Cochran, Ph.D.¥, Barry Delclos, Ph.D.∞, Daniel Doerge, Ph.D.∞, Dongyi (Tony) Du, M.D., Ph.D. ¥, Sherry Ferguson, Ph.D.∞, Jeffrey Fisher, Ph.D.∞, Suzanne Fitzpatrick, Ph.D. D.A.B.T. £, Qian Graves, Ph.D.£, Yan Gu, Ph.D.£, Ji Guo, Ph.D.¥, Deborah Hansen, Ph.D. ∞, Laura Hungerford, D.V.M., Ph.D.⌂, Nathan S Ivey, Ph.D. ¥, Abigail C Jacobs, Ph.D.∂, Elizabeth Katz, Ph.D. ¥, Hyon Kwon, Pharm.D. ∂, Ifthekar Mahmood, Ph.D. ∂, Leslie McKinney, Ph.D.∂, Robert Mitkus, Ph.D., D.A.B.T.€, Gregory Noonan, Ph.D. £, Allison O’Neill, M.A. ¥, Penelope Rice, Ph.D., D.A.B.T. £, Mary Shackelford, Ph.D. £, Evi Struble, Ph.D.€, Yelizaveta Torosyan, Ph.D. ¥, Beverly Wolpert, Ph.D.£, Hong Yang, Ph.D.€, Lisa B Yanoff, M.D.∂ *Co-Chair, € Center for Biologics Evaluation & Research, £ Center for Food Safety and Applied Nutrition, ∂ Center for Drug Evaluation and Research, ¥ Center for Devices and Radiological Health, ∞ National Center for Toxicological Research, ⌂ Center for Veterinary Medicine Subject: 2014 Updated Review of Literature and Data on Bisphenol A (CAS RN 80-05-7) To: FDA Chemical and Environmental Science Council (CESC) Office of the Commissioner Attn: Stephen M. -
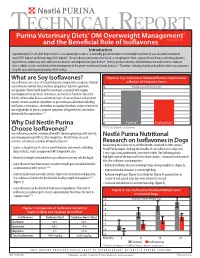
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Kellogg Company 2012 Annual Report
® Kellogg Company 2012 Annual Report ™ Pringles Rice Krispies Kashi Cheez-It Club Frosted Mini Wheats Mother’s Krave Keebler Corn Pops Pop Tarts Special K Town House Eggo Carr’s Frosted Flakes All-Bran Fudge Stripes Crunchy Nut Chips Deluxe Fiber Plus Be Natural Mini Max Zucaritas Froot Loops Tresor MorningStar Farms Sultana Bran Pop Tarts Corn Flakes Raisin Bran Apple Jacks Gardenburger Famous Amos Pringles Rice Krispies Kashi Cheez-It Club Frosted Mini Wheats Mother’s Krave Keebler Corn Pops Pop Tarts Special K Town House Eggo Carr’s Frosted Flakes All-Bran Fudge Stripes Crunchy Nut Chips Deluxe Fiber Plus Be Natural Mini Max Zucaritas Froot Loops Tresor MorningStar Farms Sultana Bran Pop Tarts Corn Flakes Raisin Bran Apple JacksCONTENTS Gardenburger Famous Amos Pringles Rice Letter to Shareowners 01 KrispiesOur Strategy Kashi Cheez-It03 Club Frosted Mini Wheats Pringles 04 Our People 06 Mother’sOur Innovations Krave Keebler11 Corn Pops Pop Tarts Financial Highlights 12 Our Brands 14 SpecialLeadership K Town House15 Eggo Carr’s Frosted Flakes Financials/Form 10-K All-BranBrands and Trademarks Fudge Stripes01 Crunchy Nut Chips Deluxe Selected Financial Data 14 FiberManagement’s Plus Discussion Be & Analysis Natural 15 Mini Max Zucaritas Froot Financial Statements 30 Notes to Financial Statements 35 LoopsShareowner Tresor Information MorningStar Farms Sultana Bran Pop Tarts Corn Flakes Raisin Bran Apple Jacks Gardenburger Famous Amos Pringles Rice Krispies Kashi Cheez-It Club Frosted Mini Wheats Mother’s Krave Keebler Corn Pops Pop Tarts Special K Town House Eggo Carr’s Frosted Flakes All-Bran Fudge Stripes Crunchy Nut Chips Deluxe Fiber Plus2 Be NaturalKellogg Company 2012 Annual Mini Report MaxMOVING FORWARD. -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

The Mysteries of the Goddess of Marmarini
Kernos Revue internationale et pluridisciplinaire de religion grecque antique 29 | 2016 Varia The Mysteries of the Goddess of Marmarini Robert Parker and Scott Scullion Electronic version URL: http://journals.openedition.org/kernos/2399 DOI: 10.4000/kernos.2399 ISSN: 2034-7871 Publisher Centre international d'étude de la religion grecque antique Printed version Date of publication: 1 October 2016 Number of pages: 209-266 ISSN: 0776-3824 Electronic reference Robert Parker and Scott Scullion, « The Mysteries of the Goddess of Marmarini », Kernos [Online], 29 | 2016, Online since 01 October 2019, connection on 10 December 2020. URL : http:// journals.openedition.org/kernos/2399 ; DOI : https://doi.org/10.4000/kernos.2399 This text was automatically generated on 10 December 2020. Kernos The Mysteries of the Goddess of Marmarini 1 The Mysteries of the Goddess of Marmarini Robert Parker and Scott Scullion For help and advice of various kinds we are very grateful to Jim Adams, Sebastian Brock, Mat Carbon, Jim Coulton, Emily Kearns, Sofia Kravaritou, Judith McKenzie, Philomen Probert, Maria Stamatopoulou, and Andreas Willi, and for encouragement to publish in Kernos Vinciane Pirenne-Delforge. Introduction 1 The interest for students of Greek religion of the large opisthographic stele published by J.C. Decourt and A. Tziafalias, with commendable speed, in the last issue of Kernos can scarcely be over-estimated.1 It is datable on palaeographic grounds to the second century BC, perhaps the first half rather than the second,2 and records in detail the rituals and rules governing the sanctuary of a goddess whose name, we believe, is never given. -

Greek Sesame Flatbread - Lagana
Recipe Category / Breads and Pastries Greek Sesame Flatbread - Lagana 20' 130' 15' 2 1 Ηands on Hands off Cook Time Portion(s) Difficulty Ingredients 500 g hard flour 350 ml water, at room temperature 10 g yeast 1 pinch granulated sugar 12 g salt 2 tablespoon(s) olive oil For coating 2 tablespoon(s) water, at room temperature 1 teaspoon(s) granulated sugar sesame seeds Διατροφικός πίνακας Nutrition information per 100 gr. Method 256 0.8 44.0 Calories 4.7 Saturated Total Carbs (kcal) Total Fat (g) Fat (g) (g) In case we don't have a mixer 13 % 7 % 4 % 17 % In a bowl add the water, the yeast, the sugar, and whisk well. Set it aside for 15-20 minutes until the yeast is activated. Once the 15 minutes pass, add the flour, the salt, and the oil into the bowl and mix with your hands until the dough is homogenized. 0.8 8.2 2.1 1.4 Do not add the salt from the beginning as it will "kill" the yeast. Sugars (g) Protein (g) Fibre (g) Sodium (g) Add a little oil into a bowl and flour your hands. Add the dough into the bowl and cover it with plastic wrap. 1 % 16 % 8 % 23 % Set it aside to rest for 30-45 minutes. Wet your hands with a little water and knead the dough into the bowl for 1-2 minutes, until it is smooth and elastic. Cover with plastic wrap and let it double in volume for 1- 1 1/2 hour.