Mutations in EFHC1 Cause Juvenile Myoclonic Epilepsy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contiguous Deletion of the NDP, MAOA, MAOB, and EFHC2 Genes in a Patient with Norrie Disease, Severe Psychomotor Retardation and Myoclonic Epilepsy
ß 2007 Wiley-Liss, Inc. American Journal of Medical Genetics Part A 143A:916–920 (2007) Contiguous Deletion of the NDP, MAOA, MAOB, and EFHC2 Genes in a Patient With Norrie Disease, Severe Psychomotor Retardation and Myoclonic Epilepsy L. Rodriguez-Revenga,1,2 I. Madrigal,1,2 L.S. Alkhalidi,3 L. Armengol,4 E. Gonza´lez,4 C. Badenas,1,2 X. Estivill,1,5 and M. Mila`1,2* 1Biochemistry and Molecular Genetics Department, Hospital Clı´nic, Barcelona, Spain 2IDIBAPS (Institut d’Investigacions Biome`diques August Pi i Sunyer), Barcelona, Spain 3Department of Health and Medical Services, Rashid Hospital, Dubai, United Arab Emirates 4Genes and Disease Programme, Centre for Genomic Regulation (CRG), Barcelona Biomedical Research Park, Barcelona, Spain 5Department of Experimental and Health Sciences, Universitat Pompeu Fabra (UPF), Barcelona, Spain Received 20 February 2006; Accepted 7 September 2006 Norrie disease (ND) is an X-linked disorder, inherited as a Clinical features of the proband include bilateral retinal recessive trait that, therefore, mostly affects males. The gene detachment, microcephaly, severe psychomotor retardation responsible for ND, called NDP, maps to the short arm of without verbal language skills acquired, and epilepsy. The chromosome X (Xp11.4-p11.3). We report here an atypical identification and molecular characterization of this case case of ND, consisting of a patient harboring a large reinforces the idea of a new contiguous gene syndrome that submicroscopic deletion affecting not only the NDP gene would explain the complex phenotype shared by atypical but also the MAOA, MAOB, and EFHC2 genes. Microarray ND patients. ß 2007 Wiley-Liss, Inc. -
Primepcr™Assay Validation Report
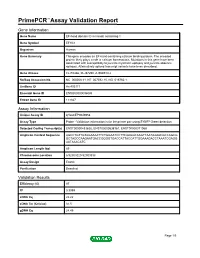
PrimePCR™Assay Validation Report Gene Information Gene Name EF-hand domain (C-terminal) containing 1 Gene Symbol EFHC1 Organism Human Gene Summary This gene encodes an EF-hand-containing calcium binding protein. The encoded protein likely plays a role in calcium homeostasis. Mutations in this gene have been associated with susceptibility to juvenile myoclonic epilepsy and juvenile absence epilepsy. Alternatively spliced transcript variants have been described. Gene Aliases FLJ10466, FLJ37290, dJ304B14.2 RefSeq Accession No. NC_000006.11, NT_007592.15, NG_016760.1 UniGene ID Hs.403171 Ensembl Gene ID ENSG00000096093 Entrez Gene ID 114327 Assay Information Unique Assay ID qHsaCEP0049958 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000433625, ENST00000538167, ENST00000371068 Amplicon Context Sequence AGCCTGTTGTAGAAAATTCTGGAATCCTTCAAGGCAAGTTAATAAAACGCCAGCG GCTAGCCAAGAATGACCGGGGTGACCATTACCATTGGAAAGACCTAAATCGAGG AATAAACATC Amplicon Length (bp) 89 Chromosome Location 6:52303220-52303338 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 97 R2 0.9999 cDNA Cq 22.22 cDNA Tm (Celsius) 81.5 gDNA Cq 24.45 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report EFHC1, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications
Stem Cell Rev and Rep DOI 10.1007/s12015-016-9662-8 Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications Behnam Ahmadian Baghbaderani 1 & Adhikarla Syama2 & Renuka Sivapatham3 & Ying Pei4 & Odity Mukherjee2 & Thomas Fellner1 & Xianmin Zeng3,4 & Mahendra S. Rao5,6 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract We have recently described manufacturing of hu- help determine which set of tests will be most useful in mon- man induced pluripotent stem cells (iPSC) master cell banks itoring the cells and establishing criteria for discarding a line. (MCB) generated by a clinically compliant process using cord blood as a starting material (Baghbaderani et al. in Stem Cell Keywords Induced pluripotent stem cells . Embryonic stem Reports, 5(4), 647–659, 2015). In this manuscript, we de- cells . Manufacturing . cGMP . Consent . Markers scribe the detailed characterization of the two iPSC clones generated using this process, including whole genome se- quencing (WGS), microarray, and comparative genomic hy- Introduction bridization (aCGH) single nucleotide polymorphism (SNP) analysis. We compare their profiles with a proposed calibra- Induced pluripotent stem cells (iPSCs) are akin to embryonic tion material and with a reporter subclone and lines made by a stem cells (ESC) [2] in their developmental potential, but dif- similar process from different donors. We believe that iPSCs fer from ESC in the starting cell used and the requirement of a are likely to be used to make multiple clinical products. We set of proteins to induce pluripotency [3]. Although function- further believe that the lines used as input material will be used ally identical, iPSCs may differ from ESC in subtle ways, at different sites and, given their immortal status, will be used including in their epigenetic profile, exposure to the environ- for many years or even decades. -

Novel Gene Discovery in Primary Ciliary Dyskinesia
Novel Gene Discovery in Primary Ciliary Dyskinesia Mahmoud Raafat Fassad Genetics and Genomic Medicine Programme Great Ormond Street Institute of Child Health University College London A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy University College London 1 Declaration I, Mahmoud Raafat Fassad, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 Abstract Primary Ciliary Dyskinesia (PCD) is one of the ‘ciliopathies’, genetic disorders affecting either cilia structure or function. PCD is a rare recessive disease caused by defective motile cilia. Affected individuals manifest with neonatal respiratory distress, chronic wet cough, upper respiratory tract problems, progressive lung disease resulting in bronchiectasis, laterality problems including heart defects and adult infertility. Early diagnosis and management are essential for better respiratory disease prognosis. PCD is a highly genetically heterogeneous disorder with causal mutations identified in 36 genes that account for the disease in about 70% of PCD cases, suggesting that additional genes remain to be discovered. Targeted next generation sequencing was used for genetic screening of a cohort of patients with confirmed or suggestive PCD diagnosis. The use of multi-gene panel sequencing yielded a high diagnostic output (> 70%) with mutations identified in known PCD genes. Over half of these mutations were novel alleles, expanding the mutation spectrum in PCD genes. The inclusion of patients from various ethnic backgrounds revealed a striking impact of ethnicity on the composition of disease alleles uncovering a significant genetic stratification of PCD in different populations. -

Meta-Analysis Identifies Seven Susceptibility Loci Involved in the Atopic March
ARTICLE Received 20 Jul 2015 | Accepted 6 Oct 2015 | Published 6 Nov 2015 DOI: 10.1038/ncomms9804 OPEN Meta-analysis identifies seven susceptibility loci involved in the atopic march Ingo Marenholz et al.# Eczema often precedes the development of asthma in a disease course called the ‘atopic march’. To unravel the genes underlying this characteristic pattern of allergic disease, we conduct a multi-stage genome-wide association study on infantile eczema followed by childhood asthma in 12 populations including 2,428 cases and 17,034 controls. Here we report two novel loci specific for the combined eczema plus asthma phenotype, which are associated with allergic disease for the first time; rs9357733 located in EFHC1 on chromo- some 6p12.3 (OR 1.27; P ¼ 2.1 Â 10 À 8) and rs993226 between TMTC2 and SLC6A15 on chromosome 12q21.3 (OR 1.58; P ¼ 5.3 Â 10 À 9). Additional susceptibility loci identified at genome-wide significance are FLG (1q21.3), IL4/KIF3A (5q31.1), AP5B1/OVOL1 (11q13.1), C11orf30/LRRC32 (11q13.5) and IKZF3 (17q21). We show that predominantly eczema loci increase the risk for the atopic march. Our findings suggest that eczema may play an important role in the development of asthma after eczema. Correspondence and requests for materials should be addressed to Y.A.L. (email: [email protected]). #A full list of authors and their affiliations appears at the end of the paper. NATURE COMMUNICATIONS | 6:8804 | DOI: 10.1038/ncomms9804 | www.nature.com/naturecommunications 1 & 2015 Macmillan Publishers Limited. All rights reserved. ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms9804 he atopic or allergic march describes the sequential located in the same region, we selected the best SNP per 1-Mb progression of different allergic conditions frequently window. -

Novel Myoclonin1/EFHC1 Mutations in Mexican Patients with Juvenile
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 21 (2012) 550–554 Contents lists available at SciVerse ScienceDirect Seizure jou rnal homepage: www.elsevier.com/locate/yseiz Short communication Novel Myoclonin1/EFHC1 mutations in Mexican patients with juvenile myoclonic epilepsy a b a a Aurelio Jara-Prado , Iris E. Martı´nez-Jua´rez , Adriana Ochoa , Vı´ctor M. Gonza´lez , c d e Marı´a del Carmen Ferna´ndez-Gonza´lez-Arago´ n , Minerva Lo´ pez-Ruiz , Marco T. Medina , f f a, Julia N. Bailey , Antonio V. Delgado-Escueta , Marı´a Elisa Alonso * a Neurogenetics and Molecular Biology Department, National Institute of Neurology and Neurosurgery of Mexico, Insurgentes Sur 3877, Col. La Fama, Tlalpan, Me´xico D.F. 14269, Mexico b Epilepsy Clinic, National Institute of Neurology and Neurosurgery of Mexico, Insurgentes Sur 3877, Col. La Fama, Tlalpan, Me´xico D.F. 14269, Mexico c Clinical Neurophysiology Department, National Institute of Neurology and Neurosurgery of Mexico, Insurgentes Sur 3877, Col. La Fama, Tlalpan, Me´xico D.F. 14269, Mexico d Neurology and Neurosurgery Unit, Mexico General Hospital, Dr. Balmis 161, Col. Doctores, Mexico D.F, Mexico e National Autonomous University of Honduras, Tegucigalpa, Honduras f Epilepsy Genetics/Genomics Laboratories and Epilepsy Center of Excellence, Neurology and Research Services, VA GLAHS and David Geffen School of Medicine at UCLA, Los Angeles, CA 90073, USA A R T I C L E I N F O A B S T R A C T Article history: Purpose: The purpose of this study was to identify the prevalence of mutations in the Myoclonin1/EFHC1 Received 1 February 2012 gene in Mexican patients with juvenile myoclonic epilepsy (JME). -

Comprehensive Epilepsy Precision Panel Overview Indications
Comprehensive Epilepsy Precision Panel Overview Epilepsy is a central nervous system disease characterized by recurrent unprovoked seizures, which are brief episodes of involuntary movement that may involve a part of the body (partial) or the entire body (generalized) and can be accompanied by loss of consciousness and loss of control of bowel or bladder function. Around 50 million people worldwide have epilepsy, making it one of the most common neurological diseases globally. Epilepsy entails an enduring predisposition to generate neurobiological, cognitive, psychological and social consequences. Multiple risk factors exist for epilepsy one of them being a strong genetic predisposition. The three major classes of epilepsy disorders are genetic generalized, focal and encephalopathic epilepsies, with several specific disorders within each class. Epilepsy genetics is shifting from an academic pursuit to a clinical discipline based on molecular diagnosis and stratified medicine. Mutations leading to epilepsy have been identified in genes encoding ion channels, neurotransmitter receptors, molecular cascade of cellular energy production and proteins involved in neuronal excitability. The mode of inheritance ranges from autosomal dominant, recessive all the way to mitochondrial. The Igenomix Comprehensive Epilepsy Precision Panel can serve as an accurate and directed diagnostic tool as well as for a differential diagnosis of recurrent seizures ultimately leading to a better management and prognosis of the disease. It provides a comprehensive -
Neurophysiological and Genetic Findings in Patients with Juvenile Myoclonic Epilepsy
fnint-14-00045 August 18, 2020 Time: 18:56 # 1 ORIGINAL RESEARCH published: 20 August 2020 doi: 10.3389/fnint.2020.00045 Neurophysiological and Genetic Findings in Patients With Juvenile Myoclonic Epilepsy Stefani Stefani1,2*, Ioanna Kousiappa1,2, Nicoletta Nicolaou3,4, Eleftherios S. Papathanasiou1,2, Anastasis Oulas1,5, Pavlos Fanis1,6, Vassos Neocleous1,6, Leonidas A. Phylactou1,6, George M. Spyrou1,5 and Savvas S. Papacostas1,2,3,4* 1 Cyprus School of Molecular Medicine, Nicosia, Cyprus, 2 Neurology Clinic B, The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus, 3 Medical School, University of Nicosia, Nicosia, Cyprus, 4 Centre for Neuroscience and Integrative Brain Research (CENIBRE), University of Nicosia, Nicosia, Cyprus, 5 Bioinformatics Group, The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus, 6 Department of Molecular Genetics, Function & Therapy, The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprus Objective: Transcranial magnetic stimulation (TMS), a non-invasive procedure, stimulates the cortex evaluating the central motor pathways. The response is called motor evoked potential (MEP). Polyphasia results when the response crosses the baseline more than twice (zero crossing). Recent research shows MEP polyphasia Edited by: in patients with generalized genetic epilepsy (GGE) and their first-degree relatives Rossella Breveglieri, University of Bologna, Italy compared with controls. Juvenile Myoclonic Epilepsy (JME), a GGE type, is not Reviewed by: well studied regarding polyphasia. In our study, we assessed polyphasia appearance Elias Manjarrez, probability with TMS in JME patients, their healthy first-degree relatives and controls. Meritorious Autonomous University Two genetic approaches were applied to uncover genetic association with polyphasia. of Puebla, Mexico Laura Säisänen, Methods: 20 JME patients, 23 first-degree relatives and 30 controls underwent TMS, Kuopio University Hospital, Finland obtaining 10–15 MEPs per participant. -

Mutational Analysis of Myoclonin1 Gene in Pakistani Juvenile Myoclonic Epilepsy Patients
Hindawi BioMed Research International Volume 2021, Article ID 7509825, 6 pages https://doi.org/10.1155/2021/7509825 Research Article Mutational Analysis of Myoclonin1 Gene in Pakistani Juvenile Myoclonic Epilepsy Patients Tayyaba Saleem , Arooj Mustafa , Nadeem Sheikh , Maryam Mukhtar , Mavra Irfan , and Saira Kainat Suqaina Cell and Molecular Biology Laboratory, Department of Zoology, University of the Punjab, Lahore 54590, Pakistan Correspondence should be addressed to Nadeem Sheikh; [email protected] Received 22 July 2020; Revised 3 February 2021; Accepted 10 April 2021; Published 21 April 2021 Academic Editor: Bilal ig Copyright © 2021 Tayyaba Saleem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Juvenile myoclonic epilepsy (JME) is the most prevalent and genetically heterogeneous form of epilepsy and accounts for 10–30% of all the cases worldwide. Ef-hand domain- (c-terminal-) containing protein 1 (EFHC1) encodes for a nonion channel protein and mutations in this gene have been extensively reported in different populations to play a causative role in JME. Linkage between JME and 6p11-12 locus has already been confirmed in Mexican and Dutch families. A case-control study was conducted on Pakistani JME patients for the first time, aimed at finding out EFHC1 mutations that have been reported in different populations. For this purpose, 66 clinically diagnosed JME patients and 108 control subjects were included in the study. Blood samples were collected from all the participants, and DNA was isolated from the lymphocytes by the modified organic method. -

You Can Check If Genes Are Captured by the Agilent Sureselect V5 Exome
IIHG Clinical Exome Sequencing Test Gene Coverage • Whole Exome Sequencing is a targeted capture platform which does not capture the entire exome. Regions not captured by the exome will not be analyzed. o Please note, it is important to understand the absence of a reportable variant in a given gene does not mean there are not pathogenic variants in that gene. • Data sensitivity and specificity for exome testing is variable as gene coverage is not uniform throughout the exome. • The Agilent SureSelect XT Human All Exon v5 kit captures ~98% of Refseq coding base pairs, and >94% of the captured coding bases in the exome are covered at our depth-of-coverage minimum threshold (30 reads). • All test reports include the following information: o Regions of the Symptom Candidate Gene List which were not captured by the current exome capture platform. o Regions of the Symptom Candidate Gene List which were not sequenced to a sufficient depth of coverage to make a clinical diagnosis. • You can check if genes are captured by the Agilent Agilent SureSelect v5 exome capture, and how well those genes are typically covered here. • Explanations for the headings; • Symbol: Gene symbol • Chr: Chromosome • % Captured: the minimum portion of the gene captured by the Agilent SureSelect XT Human All Exon v5 kit. This includes all transcripts for a given gene. o 100.00% = the entire gene is captured o 0.00% = none of the gene is captured • % Covered: the minimum portion of the gene that has at least 30x average coverage when sequenced on the Illumina HiSeq2000 with 100 base pair (bp) paired-end reads for eight CEPH samples. -

Genetic Characteristics of Non-Familial Epilepsy
Genetic characteristics of non-familial epilepsy Kyung Wook Kang1, Wonkuk Kim2, Yong Won Cho3, Sang Kun Lee4, Ki-Young Jung4, Wonchul Shin5, Dong Wook Kim6, Won-Joo Kim7, Hyang Woon Lee8, Woojun Kim9, Keuntae Kim3, So-Hyun Lee10, Seok-Yong Choi10 and Myeong-Kyu Kim1 1 Department of Neurology, Chonnam National University Medical School, Gwangju, South Korea 2 Department of Applied Statistics, Chung-Ang University, Seoul, South Korea 3 Department of Neurology, Keimyung University Dongsan Medical Center, Daegu, South Korea 4 Department of Neurology, Seoul National University Hospital, Seoul, South Korea 5 Department of Neurology, Kyung Hee University Hospital at Gangdong, Seoul, South Korea 6 Department of Neurology, Konkuk University School of Medicine, Seoul, South Korea 7 Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea 8 Department of Neurology, Ewha Womans University School of Medicine and Ewha Medical Research Institute, Seoul, South Korea 9 Department of Neurology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea 10 Department of Biomedical Science, Chonnam National University Medical School, Gwangju, South Korea ABSTRACT Background: Knowledge of the genetic etiology of epilepsy can provide essential prognostic information and influence decisions regarding treatment and management, leading us into the era of precision medicine. However, the genetic basis underlying epileptogenesis or epilepsy pharmacoresistance is not well-understood, particularly in non-familial epilepsies with heterogeneous phenotypes that last until or start in adulthood. Methods: We sought to determine the contribution of known epilepsy-associated Submitted 25 June 2019 genes (EAGs) to the causation of non-familial epilepsies with heterogeneous Accepted 22 November 2019 phenotypes and to the genetic basis underlying epilepsy pharmacoresistance.