Pegvaliase-Pqpz
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
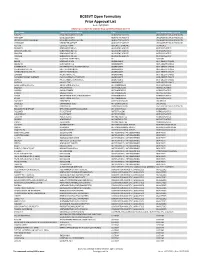
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England V14 Changes to Version 13
MEDICINES NOT REIMBURSED THROUGH NATIONAL PRICES AND DIRECTLY COMMISSIONED BY NHS ENGLAND V14 CHANGES TO VERSION 13 PUBLISHED APRIL 2019 Changes to v13 Lines removed SUITABLE SPECIALIST FOR CENTRE SUITABLE FOR SHARED ONLY SHARED CARE CARE PRIOR (includng WITH PRIMARY BETWEEN STOPPING MONITORING/ AUDIT APPROVAL outreach CARE (IF DRUG NAME INDICATION COMMISSIONER PBR CATEGORY TA/POLICY STARTING CRITERIA SPECIALIST COMMENT CRITERIA REQUIREMENTS PROFORMA when SUPPORTED AND REQUIRED delivered as BY LOCAL SECONDARY part of a PRESCRIBING CARE VIA provider COMMITTEE) NETWORK network) MODEL PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER ADULT TA'S (TA195, TA373, ABATACEPT NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE √ MEDICINES FOR CHILDREN TA375) POLICY) Now covered by comment 8 PAEDIATRIC INDICATIONS (IN AS PER TA455 OR ADULT TA'S (TA130 LINE WITH NHS ENGLAND (replaced by TA375), TA143 (repalced by ADALIMUMAB NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE AUDIT √ √ MEDICINES FOR CHILDREN TA383), TA146, TA187, TA199, TA329, POLICY) TA392, TA460) Now covered by comment 8 ALBUTREPENONACOG ALFA HAEMOPHILIA B PRODUCTS ON CMU Blood factor products simplified NHS ENGLAND BLOOD-RELATED PRODUCTS SSC 1652 SSC 1652 SSC 1652 √ FRAMEWORK to blood factor product VIII etc PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER IFR AS PER IFR ANAKINRA NHS ENGLAND CYTOKINE MODULATORS NOT ROUTINELY COMMISSIONED AS PER IFR APPROVAL √ MEDICINES FOR CHILDREN APPROVAL APPROVAL POLICY) Now covered by comment 8 AS PER BCSH GUIDELINES ANTIHAEMOPHILIC FACTOR/VON -

Specialty Pharmacy Program Drug List
Specialty Pharmacy Program Drug List The Specialty Pharmacy Program covers certain drugs commonly referred to as high-cost Specialty Drugs. To receive in- network benefits/coverage for these drugs, these drugs must be dispensed through a select group of contracted specialty pharmacies or your medical provider. Please call the BCBSLA Customer Service number on the back of your member ID card for information about our contracted specialty pharmacies. All specialty drugs listed below are limited to the retail day supply listed in your benefit plan (typically a 30-day supply). As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. Please refer to your benefit plan for a complete list of benefits, including specific exclusions, limitations and member cost-sharing amounts you are responsible for such as a deductible, copayment and coinsurance. Brand Name Generic Name Drug Classification 8-MOP methoxsalen Psoralen ACTEMRA SC tocilizumab Monoclonal Antibody/Arthritis ACTHAR corticotropin Adrenocortical Insufficiency ACTIMMUNE interferon gamma 1b Interferon ADCIRCA tadalafil Pulmonary Vasodilator ADEMPAS riociguat Pulmonary Vasodilator AFINITOR everolimus Oncology ALECENSA alectinib Oncology ALKERAN (oral) melphalan Oncology ALUNBRIG brigatinib Oncology AMPYRA ER dalfampridine Multiple Sclerosis APTIVUS tipranavir HIV/AIDS APOKYN apomorphine Parkinson's Disease ARCALYST rilonacept Interleukin Blocker/CAPS ATRIPLA efavirenz-emtricitabine-tenofovir HIV/AIDS AUBAGIO -

PALYNZIQ (Pegvaliase-Pqpz) Injection, for Subcutaneous Use Blood Phenylalanine Monitoring and Diet (2.2) Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION 600 micromol/L after 16 weeks of continuous treatment with the These highlights do not include all the information needed to use maximum dosage of 40 mg once daily. PALYNZIQ safely and effectively. See full prescribing information for Reduce the dosage and/or modify dietary protein and phenylalanine PALYNZIQ. intake, as needed, to maintain blood phenylalanine concentrations within a clinically acceptable range and above 30 micromol/L. PALYNZIQ (pegvaliase-pqpz) injection, for subcutaneous use Blood Phenylalanine Monitoring and Diet (2.2) Initial U.S. Approval: 2018 Obtain blood phenylalanine concentrations every 4 weeks until a maintenance dosage is established. WARNING: RISK OF ANAPHYLAXIS After a maintenance dosage is established, periodically monitor blood See full prescribing information for complete boxed warning. phenylalanine concentrations. Anaphylaxis has been reported after administration of Palynziq Counsel patients to monitor dietary protein and phenylalanine intake, and may occur at any time during treatment. (5.1) and adjust as directed by their healthcare provider. Administer the initial dose of Palynziq under the supervision of a Premedication (2.3, 5.1, 5.3) healthcare provider equipped to manage anaphylaxis, and closely Consider premedication for hypersensitivity reactions. observe patients for at least 60 minutes following injection. Prior Administration Instructions (2.4) to self-injection, confirm patient competency with Rotate injection sites. If more than one injection is needed for a single self-administration, and patient’s and observer’s (if applicable) dose, the injection sites should be at least 2 inches away from each other. ability to recognize signs and symptoms of anaphylaxis and to administer auto-injectable epinephrine, if needed. -

Self-Administered Specialty Drug List
Self‐Administered Drug List BRAND NAME GENERIC DRUG NME ABIRATERONE ACETATE ABIRATERONE ACETATE ACTEMRA TOCILIZUMAB ACTEMRA ACTPEN TOCILIZUMAB ACTHAR CORTICOTROPIN ACTIMMUNE INTERFERON GAMMA‐1B,RECOMB ADCIRCA TADALAFIL ADEMPAS RIOCIGUAT AFINITOR EVEROLIMUS AFINITOR DISPERZ EVEROLIMUS ALECENSA ALECTINIB HCL ALUNBRIG BRIGATINIB ALYQ TADALAFIL AMBRISENTAN AMBRISENTAN AMPYRA DALFAMPRIDINE APOKYN APOMORPHINE HCL ARANESP DARBEPOETIN ALFA IN POLYSORBAT ARCALYST RILONACEPT ARIKAYCE AMIKACIN LIPOSOMAL/NEB.ACCESSR ARIXTRA FONDAPARINUX SODIUM AUBAGIO TERIFLUNOMIDE AUSTEDO DEUTETRABENAZINE AVEED TESTOSTERONE UNDECANOATE AVONEX INTERFERON BETA‐1A AVONEX INTERFERON BETA‐1A/ALBUMIN AVONEX PEN INTERFERON BETA‐1A AYVAKIT AVAPRITINIB BAFIERTAM MONOMETHYL FUMARATE BALVERSA ERDAFITINIB BENLYSTA BELIMUMAB BETASERON INTERFERON BETA‐1B BETHKIS TOBRAMYCIN BOSENTAN BOSENTAN BOSULIF BOSUTINIB BRAFTOVI ENCORAFENIB BRONCHITOL MANNITOL BRUKINSA ZANUBRUTINIB BYNFEZIA OCTREOTIDE ACETATE CABENUVA CABOTEGRAVIR/RILPIVIRINE CABLIVI CAPLACIZUMAB‐YHDP CABOMETYX CABOZANTINIB S‐MALATE CALQUENCE ACALABRUTINIB CAPECITABINE CAPECITABINE CAPRELSA VANDETANIB CARBAGLU CARGLUMIC ACID CAYSTON AZTREONAM LYSINE CERDELGA ELIGLUSTAT TARTRATE CETROTIDE CETRORELIX ACETATE CHENODAL CHENODIOL CHOLBAM CHOLIC ACID CHORIONIC GONADOTROPIN CHORIONIC GONADOTROPIN, HUMAN CIMZIA CERTOLIZUMAB PEGOL COPAXONE GLATIRAMER ACETATE COPIKTRA DUVELISIB COSENTYX (2 SYRINGES) SECUKINUMAB COSENTYX PEN SECUKINUMAB COSENTYX PEN (2 PENS) SECUKINUMAB COSENTYX SYRINGE SECUKINUMAB COTELLIC COBIMETINIB FUMARATE CYSTADANE -
Synlogic DESIGNED for LIFE
Synlogic DESIGNED FOR LIFE 2019 Wedbush PacGrow Healthcare Conference Scott Plevy, MD, Chief Scientific Officer August 13, 2019 ©© 20192019 SYNLOGIC.SYNLOGIC. ALL RIGHTS RESERVED. | 1 Forward Looking Statements This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this presentation regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward-looking statements. In addition, when or if used in this presentation, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, the approach we are taking to discover and develop novel therapeutics using synthetic biology; statements regarding the potential of our platform to develop therapeutics to address a wide range of diseases, including: inborn errors of metabolism, liver disease, inflammatory and immune disorders, and cancer; the future clinical development of Synthetic Biotic medicines; the potential of our technology to treat hyperammonemia and phenylketonuria; the expected timing of our anticipated clinical trial initiations; the benefit of orphan drug and fast track status; -

Ideas and Incentives for Advanced Manufacturing
Packaging Solubility Global JANUARY 2019 Volume 43 Number 1 PLUS: Trends Testing Inspections Ideas and Incentives for Advanced Manufacturing PEER-REVIEWED Evaluating a New Quality Control Test for Soft Gelatin Rectal Capsules FORMULATION SPECIAL REPORT BIOMANUFACTURING Protein and Peptide Delivery Employment Survey Virus Filtration the next bioavailability challenge... Let’s solve it together. Dissolution rate and solubility issues are With our deep knowledge, broad expertise, Visit pharma.lonza.com increasingly common for drug candidates. We and established track record we can find the USA +1 800 706 8655 can help you optimize the bioavailability of your right solution to your specific challenge. Rest of world +44 (0)1 506 448080 compound through our comprehensive toolbox Email [email protected] of enabling technologies. Our approaches Advance your compound from concept to include API crystal structure design, particle commercialization with one partner – Lonza. © 2018 Lonza. All rights reserved. size reduction, amorphous dispersions, and lipid-based formulations. Confidence in managing your path to compliance From buying to applying….Only USP official Reference Standards come with the added value you need to help achieve compliance and product specifications with confidence. The sheer breadth of standards and depth of knowledge we provide helps mitigate the risks you might encounter on your journey to compliance. It’s our core focus. Talk to USP about Reference Standards and find out how we can help navigate the path. usp.org/confidence-pharma -

And Consensus-Based Recommendations for the Use of Pegvaliase in Adults with Phenylketonuria
SPECIAL ARTICLE Evidence- and consensus-based recommendations for the use of pegvaliase in adults with phenylketonuria Nicola Longo, MD, PhD 1, David Dimmock, MD2, Harvey Levy, MD3, Krista Viau, PhD, RD3, Heather Bausell, RD, LDN4, Deborah A. Bilder, MD5, Barbara Burton, MD6, Christel Gross, RN7, Hope Northrup, MD8, Fran Rohr, MS, RD9, Stephanie Sacharow, MD3, Amarilis Sanchez-Valle, MD10, Mary Stuy, MD11, Janet Thomas, MD12, Jerry Vockley, MD, PhD13, Roberto Zori, MD14 and Cary O. Harding, MD15 Purpose: Phenylketonuria (PKU) is a rare metabolic disorder that total of 34 guidance statements were included in the modified requires life-long management to reduce phenylalanine (Phe) Delphi voting and consensus was reached on all after two rounds of concentrations within the recommended range. The availability of voting. ™ pegvaliase (PALYNZIQ , an enzyme that can metabolize Phe) as a Conclusion: Here we describe evidence- and consensus-based new therapy necessitates the provision of guidance for its use. recommendations for the use of pegvaliase in adults with PKU. The Methods: A Steering Committee comprising 17 health-care manuscript was evaluated against the Appraisal of Guidelines for professionals with experience in using pegvaliase through the Research and Evaluation (AGREE II) instrument and is intended clinical development program drafted guidance statements during a for use by health-care professionals who will prescribe pegvaliase series of face-to-face meetings. A modified Delphi methodology was and those who will treat patients receiving pegvaliase. used to demonstrate consensus among a wider group of health-care professionals with experience in using pegvaliase. Genetics in Medicine (2019) 21:1851–1867; https://doi.org/10.1038/s41436- Results: Guidance statements were developed for four categories: 018-0403-z (1) treatment goals and considerations prior to initiating therapy, (2) dosing considerations, (3) considerations for dietary manage- Keywords: anaphylaxis management; dietary management; ment, and (4) best approaches to optimize medical management. -

(PKU) Patients on Pegvaliase
Molecular Genetics and Metabolism Reports 28 (2021) 100771 Contents lists available at ScienceDirect Molecular Genetics and Metabolism Reports journal homepage: www.elsevier.com/locate/ymgmr Development of a practical dietitian road map for the nutritional management of phenylketonuria (PKU) patients on pegvaliase Júlio C´esar Rocha a,b,c,*, Heather Bausell d, Amaya B´elanger-Quintana e, Laurie Bernstein f, ¨ g h i j k Hülya Gokmen-¨ Ozel , Alexandra Jung , Anita MacDonald , Fran Rohr , Esther van Dam , Margret Heddrich-Ellerbrok l a Nutrition & Metabolism, NOVA Medical School, Faculty of Medical Sciences, University of Lisbon, Lisbon, Portugal b Reference Centre of Inherited Metabolic Diseases, Centro Hospitalar Universitario´ de Lisboa Central, Lisbon, Portugal c Center for Health Technology and Services Research (CINTESIS), Porto, Portugal d Division of Clinical Nutrition & Genetics, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, USA e Metabolic Disease Unit, Hospital Ramon y Cajal, Madrid, Spain f Department of Pediatrics Section of Clinical Genetics and Metabolism, Aurora, Children's Hospital Colorado, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA g Nutrition and Dietetics Department, Faculty of Health Sciences, Hacettepe University, Ankara, Turkey h Competence Center for Rare Metabolic Diseases, Charit´e – University Hospital Berlin, Berlin, Germany i Department of Dietetics, Birmingham Women's and Children's Hospital, Birmingham, UK j Met Ed, Boulder, CO, USA k Department of Dietetics, University of Groningen, University Medical Center Groningen, Beatrix Children's Hospital, Groningen, The Netherlands l University Children's Hospital, University Medical Center Hamburg-Eppendorf, Hamburg, Germany ARTICLE INFO ABSTRACT Keywords: Background: The metabolic dietitian/nutritionist (hereafter ‘dietitian’) plays an essential role in the nutritional Phenylketonuria management of patients with phenylketonuria (PKU), including those on pegvaliase.