Synlogic DESIGNED for LIFE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperammonemia in Review: Pathophysiology, Diagnosis, and Treatment
Pediatr Nephrol DOI 10.1007/s00467-011-1838-5 EDUCATIONAL REVIEW Hyperammonemia in review: pathophysiology, diagnosis, and treatment Ari Auron & Patrick D. Brophy Received: 23 September 2010 /Revised: 9 January 2011 /Accepted: 12 January 2011 # IPNA 2011 Abstract Ammonia is an important source of nitrogen and is the breakdown and catabolism of dietary and bodily proteins, required for amino acid synthesis. It is also necessary for respectively. In healthy individuals, amino acids that are not normal acid-base balance. When present in high concentra- needed for protein synthesis are metabolized in various tions, ammonia is toxic. Endogenous ammonia intoxication chemical pathways, with the rest of the nitrogen waste being can occur when there is impaired capacity of the body to converted to urea. Ammonia is important for normal animal excrete nitrogenous waste, as seen with congenital enzymatic acid-base balance. During exercise, ammonia is produced in deficiencies. A variety of environmental causes and medica- skeletal muscle from deamination of adenosine monophos- tions may also lead to ammonia toxicity. Hyperammonemia phate and amino acid catabolism. In the brain, the latter refers to a clinical condition associated with elevated processes plus the activity of glutamate dehydrogenase ammonia levels manifested by a variety of symptoms and mediate ammonia production. After formation of ammonium signs, including significant central nervous system (CNS) from glutamine, α-ketoglutarate, a byproduct, may be abnormalities. Appropriate and timely management requires a degraded to produce two molecules of bicarbonate, which solid understanding of the fundamental pathophysiology, are then available to buffer acids produced by dietary sources. differential diagnosis, and treatment approaches available. -

Effect of Propionic Acid on Fatty Acid Oxidation and U Reagenesis
Pediat. Res. 10: 683- 686 (1976) Fatty degeneration propionic acid hyperammonemia propionic acidemia liver ureagenesls Effect of Propionic Acid on Fatty Acid Oxidation and U reagenesis ALLEN M. GLASGOW(23) AND H. PET ER C HASE UniversilY of Colorado Medical Celller, B. F. SlOlillsky LaboralOries , Denver, Colorado, USA Extract phosphate-buffered salin e, harvested with a brief treatment wi th tryps in- EDTA, washed twice with ph os ph ate-buffered saline, and Propionic acid significantly inhibited "CO z production from then suspended in ph os ph ate-buffe red saline (145 m M N a, 4.15 [I-"ejpalmitate at a concentration of 10 11 M in control fibroblasts m M K, 140 m M c/, 9.36 m M PO" pH 7.4) . I n mos t cases the cells and 100 11M in methyl malonic fibroblasts. This inhibition was we re incubated in 3 ml phosph ate-bu ffered sa lin e cont aining 0.5 similar to that produced by 4-pentenoic acid. Methylmalonic acid I1Ci ll-I4Cj palm it ate (19), final concentration approximately 3 11M also inhibited ' 'C0 2 production from [V 'ejpalmitate, but only at a added in 10 II I hexane. Increasing the amount of hexane to 100 II I concentration of I mM in control cells and 5 mM in methyl malonic did not impair palmit ate ox id ation. In two experiments (Fig. 3) the cells. fibroblasts were in cub ated in 3 ml calcium-free Krebs-Ringer Propionic acid (5 mM) also inhibited ureagenesis in rat liver phosphate buffer (2) co nt ain in g 5 g/ 100 ml essent iall y fatty ac id slices when ammonia was the substrate but not with aspartate and free bovine se rum albumin (20), I mM pa lm itate, and the same citrulline as substrates. -
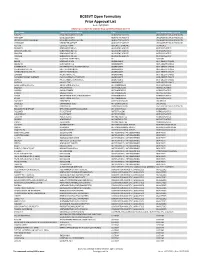
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Propionic Acidemia Information for Health Professionals
Propionic Acidemia Information for Health Professionals Propionic acidemia is an organic acid disorder in which individuals are lacking or have reduced activity of the enzyme propionyl-CoA carboxylase, leading to propionic acidemia. Clinical Symptoms Symptoms generally begin in the first few days following birth. Metabolic crisis can occur, particularly after fasting, periods of illness/infection, high protein intake, or during periods of stress on the body. Symptoms of a metabolic crisis include lethargy, behavior changes, feeding problems, hypotonia, and vomiting. If untreated, metabolic crises can lead to tachypnea, brain swelling, cardiomyopathy, seizures, coma, basal ganglia stroke, and death. Many babies die within the first year of life. Lab findings during a metabolic crisis commonly include urine ketones, hyperammonemia, metabolic acidosis, low platelets, low white blood cells, and high blood ammonia and glycine levels. Long term effects may occur despite treatment and include developmental delay, brain damage, dystonia, failure to thrive, short stature, spasticity, pancreatitis, osteoporosis, and skin lesions. Incidence Propionic acidemia occurs in greater than 1 in 75,000 live births and is more common in Saudi Arabians and the Inuit population of Greenland. Genetics of propionic acidemia Mutations in the PCCA and PCCB genes cause propionic acidemia. Mutations prevent the production of or reduce the activity of propionyl-CoA carboxylase, which converts propionyl-CoA to methylmalonyl-CoA. This causes the body to be unable to correctly process isoleucine, valine, methionine, and threonine, resulting in an accumulation of glycine and propionic acid, which causes the symptoms seen in this condition. How do people inherit propionic acidemia? Propionic acidemia is inherited in an autosomal recessive manner. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Arginine-Provider-Fact-Sheet.Pdf
Arginine (Urea Cycle Disorder) Screening Fact Sheet for Health Care Providers Newborn Screening Program of the Oklahoma State Department of Health What is the differential diagnosis? Argininemia (arginase deficiency, hyperargininemia) What are the characteristics of argininemia? Disorders of arginine metabolism are included in a larger group of disorders, known as urea cycle disorders. Argininemia is an autosomal recessive inborn error of metabolism caused by a defect in the final step in the urea cycle. Most infants are born to parents who are both unknowingly asymptomatic carriers and have NO known history of a urea cycle disorder in their family. The incidence of all urea cycle disorders is estimated to be about 1:8,000 live births. The true incidence of argininemia is not known, but has been estimated between 1:350,000 and 1:1,000,000. Argininemia is usually asymptomatic in the neonatal period, although it can present with mild to moderate hyperammonemia. Untreated, argininemia usually progresses to severe spasticity, loss of ambulation, severe cognitive and intellectual disabilities and seizures Lifelong treatment includes a special diet, and special care during times of illness or stress. What is the screening methodology for argininemia? 1. An amino acid profile by Tandem Mass Spectrometry (MS/MS) is performed on each filter paper. 2. Arginine is the primary analyte. What is an in-range (normal) screen result for arginine? Arginine less than 100 mol/L is NOT consistent with argininemia. See Table 1. TABLE 1. In-range Arginine Newborn Screening Results What is an out-of-range (abnormal) screen for arginine? Arginine > 100 mol/L requires further testing. -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England V14 Changes to Version 13
MEDICINES NOT REIMBURSED THROUGH NATIONAL PRICES AND DIRECTLY COMMISSIONED BY NHS ENGLAND V14 CHANGES TO VERSION 13 PUBLISHED APRIL 2019 Changes to v13 Lines removed SUITABLE SPECIALIST FOR CENTRE SUITABLE FOR SHARED ONLY SHARED CARE CARE PRIOR (includng WITH PRIMARY BETWEEN STOPPING MONITORING/ AUDIT APPROVAL outreach CARE (IF DRUG NAME INDICATION COMMISSIONER PBR CATEGORY TA/POLICY STARTING CRITERIA SPECIALIST COMMENT CRITERIA REQUIREMENTS PROFORMA when SUPPORTED AND REQUIRED delivered as BY LOCAL SECONDARY part of a PRESCRIBING CARE VIA provider COMMITTEE) NETWORK network) MODEL PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER ADULT TA'S (TA195, TA373, ABATACEPT NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE √ MEDICINES FOR CHILDREN TA375) POLICY) Now covered by comment 8 PAEDIATRIC INDICATIONS (IN AS PER TA455 OR ADULT TA'S (TA130 LINE WITH NHS ENGLAND (replaced by TA375), TA143 (repalced by ADALIMUMAB NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE AUDIT √ √ MEDICINES FOR CHILDREN TA383), TA146, TA187, TA199, TA329, POLICY) TA392, TA460) Now covered by comment 8 ALBUTREPENONACOG ALFA HAEMOPHILIA B PRODUCTS ON CMU Blood factor products simplified NHS ENGLAND BLOOD-RELATED PRODUCTS SSC 1652 SSC 1652 SSC 1652 √ FRAMEWORK to blood factor product VIII etc PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER IFR AS PER IFR ANAKINRA NHS ENGLAND CYTOKINE MODULATORS NOT ROUTINELY COMMISSIONED AS PER IFR APPROVAL √ MEDICINES FOR CHILDREN APPROVAL APPROVAL POLICY) Now covered by comment 8 AS PER BCSH GUIDELINES ANTIHAEMOPHILIC FACTOR/VON -

The Pharmabiotic for Phenylketonuria: Development of a Novel Therapeutic
University of South Carolina Scholar Commons Senior Theses Honors College Spring 2019 The hP armabiotic for Phenylketonuria: Development of a Novel Therapeutic Chloé Elizabeth LeBegue University of South Carolina, [email protected] Follow this and additional works at: https://scholarcommons.sc.edu/senior_theses Part of the Alternative and Complementary Medicine Commons, Amino Acids, Peptides, and Proteins Commons, Biochemical Phenomena, Metabolism, and Nutrition Commons, Biotechnology Commons, Congenital, Hereditary, and Neonatal Diseases and Abnormalities Commons, Digestive, Oral, and Skin Physiology Commons, Digestive System Diseases Commons, Enzymes and Coenzymes Commons, Genetic Phenomena Commons, Medical Biotechnology Commons, Medical Genetics Commons, Medical Nutrition Commons, Medical Pharmacology Commons, Nutritional and Metabolic Diseases Commons, Other Pharmacy and Pharmaceutical Sciences Commons, Pharmaceutical Preparations Commons, Pharmaceutics and Drug Design Commons, and the Preventive Medicine Commons Recommended Citation LeBegue, Chloé Elizabeth, "The hP armabiotic for Phenylketonuria: Development of a Novel Therapeutic" (2019). Senior Theses. 267. https://scholarcommons.sc.edu/senior_theses/267 This Thesis is brought to you by the Honors College at Scholar Commons. It has been accepted for inclusion in Senior Theses by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. THE PHARMABIOTIC FOR PHENYLKETONURIA: DEVELOPMENT OF A NOVEL THERAPEUTIC By Chloé Elizabeth -

Fatal Propionic Acidemia: a Challenging Diagnosis
Issue: Ir Med J; Vol 112; No. 7; P980 Fatal Propionic Acidemia: A Challenging Diagnosis A. Fulmali, N. Goggin 1. Department of Paediatrics, NDDH, Barnstaple, UK 2. Department of Paediatrics, UHW, Waterford, Ireland Dear Sir, We present a two days old neonate with severe form of propionic acidemia with lethal outcome. Propionic acidemia is an AR disorder, presents in the early neonatal period with progressive encephalopathy and death can occur quickly. A term neonate admitted to NICU on day 2 with poor feeding, lethargy and dehydration. Parents are non- consanguineous and there was no significant family history. Prenatal care had been excellent. Delivery had been uneventful. No resuscitation required with good APGAR scores. Baby had poor suck, lethargy, hypotonia and had lost about 13% of the birth weight. Initial investigations showed hypoglycemia (2.3mmol/L), uremia (8.3mmol/L), hypernatremia (149 mmol/L), severe metabolic acidosis (pH 7.24, HCO3 9.5, BE -18.9) with high anion gap (41) and ketonuria (4+). Hematologic parameters, inflammatory markers and CSF examination were unremarkable. Baby received initial fluid resuscitation and commenced on IV antibiotics. Generalised seizures became eminent at 70 hours of age. Loading doses of phenobarbitone and phenytoin were given. Hepatomegaly of 4cm was spotted on day 4 of life. Very soon baby became encephalopathic requiring invasive ventilation. At this stage clinical features were concerning for metabolic disorder and hence was transferred to tertiary care centre where further investigations showed high ammonia level (1178 μg/dl) and urinary organic acids were suggestive of propionic acidemia. Specific treatment for hyperammonemia and propionic acidemia was started. -

Pegvaliase-Pqpz
Pharmacy Benefit Coverage Criteria Effective Date………….………….....................12/1/2020 Next Review Date………………………………. 12/1/2021 Coverage Policy Number ................................ P0055 Pegvaliase-pqpz Table of Contents Related Coverage Resources Medical Necessity Criteria ................................... 1 Sapropterin FDA Approved Indications ................................... 2 Recommended Dosing ........................................ 2 General Background ............................................ 3 References .......................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s