Serve You Rx Prior Authorization Information and Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
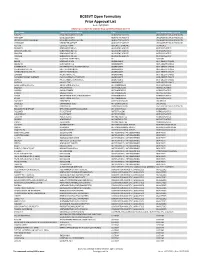
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Minutes of the CHMP Meeting 14-17 September 2020
13 January 2021 EMA/CHMP/625456/2020 Corr.1 Human Medicines Division Committee for medicinal products for human use (CHMP) Minutes for the meeting on 14-17 September 2020 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes Disclaimers Some of the information contained in these minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, these minutes are a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). 1 Addition of the list of participants Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. -

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED)
Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED) A Abatacept (ORENCIA) Abemaciclib (VERZENIO) Abiraterone Acetate (ZYTIGA) Abiraterone Acetate (+ GENERIC FORMULATIONS) AbobotulinumtoxinA (DYSPORT) Abraxane (ABRAXANE) Acalabrutinib (CALQUENCE) Actemra (ACTEMRA) Adalimumab (AMGEVITA) Adalimumab (HADLIMA) Adalimumab (HULIO) Adalimumab (HUMIRA) Adalimumab (HYRIMOZ) Adalimumab (IDACIO) Adcetris (ADCETRIS) Adcirca (ADCIRCA) Adempas (ADEMPAS) Afatinib (GIOTRIF) Afinitor (AFINITOR) Aflibercept (EYLEA) Aflibercept (ZALTRAP) Afstyla (AFSTYLA) Agalsidase Alfa (REPLAGAL) Agalsidase Beta (FABRAZYM) Aimovig (AIMOVIG) Ajovy (FREMANEZUMAB) Aldesleukin (PROLEUKIN) Aldurazyme (ALDURAZYME) Alecensaro (ALECENSARO) Alectinib (ALECENSARO) Alefacept (AMEVIVE) Alemtuzumab (MABCAMPATH) Alemtuzumab (LEMTRADA) Alglucosidase Alfa (MYOZYME) Alimta (ALIMTA) Alirocumab (PRALUENT) Alpha-1-Proteinase Inhibitor (PROLASTIN - C) Alpha-1-Proteinase Inhibitor (ZEMAIRA) Alpelisib (PIQRAY) Alunbrig (ALUNBRIG) Ambrisentan (VOLIBRIS) Ambrisentan (Generic Formulations) Amevive (AMEVIVE) Amgevita (ADALIMUMAB) Amifampridine Phosphate (FIRDAPSE) Amifampridine Phosphate (RUZURGI) Anakinra (KINERET) Apalutamide (ERLEADA) Apomorphine (KYNMOBI) Apremilast (OTEZLA) Page 1 of 17 Updated: May 20, 2021 Arsenic Trioxide (TRISENOX) Arsenic Trioxide (Generic Formulations) Arzerra (ARZERRA) Asfotase Alfa (STRENSIQ) Asparaginase (ERWINASE) Asunaprevir (SUNVEPRA) Atezolizumab (TECENTRIQ) Atriance (ATRIANCE) Aubagio (AUBAGIO) Avastin (AVASTIN) Avelumab (BAVENCIO) Avonex -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

761094Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761094Orig1s000 CLINICAL REVIEW(S) Medical Officer’s Review of BLA 761094 Review #2 BLA 761094 Submission Date: July 19, 2018 Receipt Date: July 19, 2018 Review Date: July 23, 2018 Applicant: Dompé farmaceutici S.p.A. Via Santa Lucia 6-20122 Milan, Italy Applicant’s Representative: Lamberto Dionigi. Regulatory Affairs & Drug Safety Director 39-02-5838-3559 Drug: OXERVATE (cenegermin – bkbj) ophthalmic solution, 20 mcg/mL Submitted: Reference is made to the meeting Teleconference of 15-July-2018, in which the FDA has requested to provide information available at Dompé on the use of Oxervate in the pediatric population. Pediatric patients exposed to Oxervate: Dompé confirms that Oxervate is not yet approved in Europe for use in children and no clinical studies have been conducted in this population. As far as the company is aware, four (4) Neurotrophic Keratitis (NK) pediatric patients, aged from 2 to 12 years of age, have been exposed to Oxervate. A 6-year-old child with severe NK secondary to Stuve-Wiedmann syndrome was treated with Oxervate under “Temporary Authorization for Use” (ATU) in France. Dompé reports that the corneal ulcer was completely healed at the end of the treatment. There were signs of corneal sensitivity recovery by month 8 post-treatment. A 9-year-old child with NK was treated with Oxervate under ATU in France. Dompé reports that the lesion improved, but did not completely heal with the treatment. There is no other follow-up information available. A 12-year-old child with severe NK was treated with Oxervate under Expanded Access in the US. -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Spotlight on Market Access Actionable Understandings from AIS Health’S In-Depth Coverage
Spotlight on Market Access Actionable understandings from AIS Health’s in-depth coverage September 16, 2019 Recent Situations Stress That Data Is More Important Than Ever to FDA, Drug Uptake 2 Clinical Trials by Indication: Q2 2019 A pair of drugmakers and the FDA found themselves in the news lately, but it’s safe to say it wasn’t for the reasons they would prefer. Both situations Reality Check: PCSK9 Inhibitors 8 stress the importance of data needed to secure product approvals, and, per- haps, payer and provider uptake. On Aug. 6, the FDA put out a statement addressing “data accuracy issues” with Zolgensma (onasemnogene abeparvovec-xioi), a new gene ther- apy to treat spinal muscular atrophy in people less than 2 years old who have bi-allelic mutations in the survival motor neuron 1 gene, including those who are presymptomatic when diagnosed. The one-time therapy has the distinction of being the most expensive drug in the world, with a price tag of $2.1 million. The FDA approved the drug from AveXis, Inc. on May 24 (SMA 7/1/19, p. 6). On June 28, AveXis — which was acquired by Novartis AG last year — notified the agency that there was “a data manipulation issue that impacts the accuracy of certain data from product testing performed in animals sub- mitted in the biologics license application (BLA) and reviewed by the FDA.” continued on p. 3 Report Reveals That Almost 100 RM/AT Products Are in Phase III Clinical Trials This past quarter saw two new gene therapies: Novartis AG subsidiary AveXis, Inc.’s Zolgensma (onasemnogene abeparvovec-xioi) received FDA approval May 24 for the treatment of spinal muscular atrophy (SMA 7/1/19, p. -

Horizon Therapeutics Public Annual Report 2020
Horizon Therapeutics Public Annual Report 2020 Form 10-K (NASDAQ:HZNP) Published: February 26th, 2020 PDF generated by stocklight.com octb inte UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 001-35238 HORIZON THERAPEUTICS PUBLIC LIMITED COMPANY (Exact name of Registrant as specified in its charter) Ireland Not Applicable (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) Connaught House, 1st Floor 1 Burlington Road, Dublin 4, D04 C5Y6, Ireland Not Applicable (Address of principal executive offices) (Zip Code) 011 353 1 772 2100 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol Name of Each Exchange on Which Registered Ordinary shares, nominal value $0.0001 per share HZNP The Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐. Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒. Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.