Appraisal of NICE's Cost-Effectiveness Thresholds for Assessing Orphan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
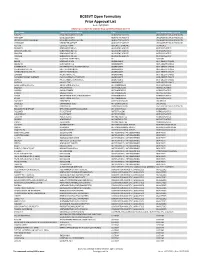
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Clofazimine As a Treatment for Multidrug-Resistant Tuberculosis: a Review
Scientia Pharmaceutica Review Clofazimine as a Treatment for Multidrug-Resistant Tuberculosis: A Review Rhea Veda Nugraha 1 , Vycke Yunivita 2 , Prayudi Santoso 3, Rob E. Aarnoutse 4 and Rovina Ruslami 2,* 1 Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung 40161, Indonesia; [email protected] 2 Division of Pharmacology and Therapy, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung 40161, Indonesia; [email protected] 3 Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin Hospital, Bandung 40161, Indonesia; [email protected] 4 Department of Pharmacy, Radboud University Medical Center, Radboud Institute for Health Sciences, 6255HB Nijmegen, The Netherlands; [email protected] * Correspondence: [email protected] Abstract: Multidrug-resistant tuberculosis (MDR-TB) is an infectious disease caused by Mycobac- terium tuberculosis which is resistant to at least isoniazid and rifampicin. This disease is a worldwide threat and complicates the control of tuberculosis (TB). Long treatment duration, a combination of several drugs, and the adverse effects of these drugs are the factors that play a role in the poor outcomes of MDR-TB patients. There have been many studies with repurposed drugs to improve MDR-TB outcomes, including clofazimine. Clofazimine recently moved from group 5 to group B of drugs that are used to treat MDR-TB. This drug belongs to the riminophenazine class, which has lipophilic characteristics and was previously discovered to treat TB and approved for leprosy. This review discusses the role of clofazimine as a treatment component in patients with MDR-TB, and Citation: Nugraha, R.V.; Yunivita, V.; the drug’s properties. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Horizon Therapeutics Public Annual Report 2020
Horizon Therapeutics Public Annual Report 2020 Form 10-K (NASDAQ:HZNP) Published: February 26th, 2020 PDF generated by stocklight.com octb inte UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 001-35238 HORIZON THERAPEUTICS PUBLIC LIMITED COMPANY (Exact name of Registrant as specified in its charter) Ireland Not Applicable (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) Connaught House, 1st Floor 1 Burlington Road, Dublin 4, D04 C5Y6, Ireland Not Applicable (Address of principal executive offices) (Zip Code) 011 353 1 772 2100 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol Name of Each Exchange on Which Registered Ordinary shares, nominal value $0.0001 per share HZNP The Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐. Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒. Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England V14 Changes to Version 13
MEDICINES NOT REIMBURSED THROUGH NATIONAL PRICES AND DIRECTLY COMMISSIONED BY NHS ENGLAND V14 CHANGES TO VERSION 13 PUBLISHED APRIL 2019 Changes to v13 Lines removed SUITABLE SPECIALIST FOR CENTRE SUITABLE FOR SHARED ONLY SHARED CARE CARE PRIOR (includng WITH PRIMARY BETWEEN STOPPING MONITORING/ AUDIT APPROVAL outreach CARE (IF DRUG NAME INDICATION COMMISSIONER PBR CATEGORY TA/POLICY STARTING CRITERIA SPECIALIST COMMENT CRITERIA REQUIREMENTS PROFORMA when SUPPORTED AND REQUIRED delivered as BY LOCAL SECONDARY part of a PRESCRIBING CARE VIA provider COMMITTEE) NETWORK network) MODEL PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER ADULT TA'S (TA195, TA373, ABATACEPT NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE √ MEDICINES FOR CHILDREN TA375) POLICY) Now covered by comment 8 PAEDIATRIC INDICATIONS (IN AS PER TA455 OR ADULT TA'S (TA130 LINE WITH NHS ENGLAND (replaced by TA375), TA143 (repalced by ADALIMUMAB NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE AUDIT √ √ MEDICINES FOR CHILDREN TA383), TA146, TA187, TA199, TA329, POLICY) TA392, TA460) Now covered by comment 8 ALBUTREPENONACOG ALFA HAEMOPHILIA B PRODUCTS ON CMU Blood factor products simplified NHS ENGLAND BLOOD-RELATED PRODUCTS SSC 1652 SSC 1652 SSC 1652 √ FRAMEWORK to blood factor product VIII etc PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER IFR AS PER IFR ANAKINRA NHS ENGLAND CYTOKINE MODULATORS NOT ROUTINELY COMMISSIONED AS PER IFR APPROVAL √ MEDICINES FOR CHILDREN APPROVAL APPROVAL POLICY) Now covered by comment 8 AS PER BCSH GUIDELINES ANTIHAEMOPHILIC FACTOR/VON -

Orphan Drugs Used for Treatment in Pediatric Patients in the Slovak Republic
DOI 10.2478/v10219-012-0001-0 ACTA FACULTATIS PHARMACEUTICAE UNIVERSITATIS COMENIANAE Supplementum VI 2012 ORPHAN DRUGS USED FOR TREATMENT IN PEDIATRIC PATIENTS IN THE SLOVAK REPUBLIC 1Foltánová, T. – 2Konečný, M. – 3Hlavatá, A. –.4Štepánková, K. 5Cisárik, F. 1Comenius University in Bratislava, Faculty of Pharmacy, Department of Pharmacology and Toxicology 2Department of Clinical Genetics, St. Elizabeth Cancer Institute, Bratislava 32nd Department of Pediatrics, UniversityChildren'sHospital, Bratislava 4Slovak Cystic Fibrosis Association, Košice 5Department of Medical Genetics, Faculty Hospital, Žilina Due to the enormous success of scientific research in the field of paediatric medicine many once fatal children’s diseases can now be cured. Great progress has also been achieved in the rehabilitation of disabilities. However, there is still a big group of diseases defined as rare, treatment of which has been traditionally neglected by the drug companies mainly due to unprofitability. Since 2000 the treatment of rare diseases has been supported at the European level and in 2007 paediatric legislation was introduced. Both decisions together support treatment of rare diseases in children. In this paper, we shortly characterise the possibilities of rare diseases treatment in children in the Slovak republic and bring the list of orphan medicine products (OMPs) with defined dosing in paediatrics, which were launched in the Slovak market. We also bring a list of OMPs with defined dosing in children, which are not available in the national market. This incentive may help in further formation of the national plan for treating rare diseases as well as improvement in treatment options and availability of rare disease treatment in children in Slovakia. -

Primechoice Accord Formulary, January 2019 I 2018
January 2019 PrimeChoice Accord Formulary Please consider talking to your doctor about prescribing preferred medications, which may help reduce your out-of-pocket costs. This list may help guide you and your doctor in selecting an appropriate medication for you. The drug list, also known as a formulary, is regularly updated. You can view the most up-to-date list, or the specialty drug list, at MyPrime.com. Contents Therapeutic Class Drug List Introduction ...................................................................... I Anti-Infective Agents ....................................................... 1 Coverage considerations ................................................. I Biologicals ....................................................................... 5 Abbreviation/acronym key ............................................... I Antineoplastic Agents ..................................................... 5 Endocrine and Metabolic Drugs ...................................... 7 Cardiovascular Agents .................................................. 13 Respiratory Agents ....................................................... 19 Gastrointestinal Agents ................................................. 21 Genitourinary Agents .................................................... 23 Central Nervous System Drugs .................................... 24 Analgesics and Anesthetics .......................................... 29 Neuromuscular Drugs ................................................... 32 Nutritional Products...................................................... -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L
PEDIATRIC PHARMACOTHERAPY Volume 21 Number 11 November 2015 New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L. Buck, PharmD, FCCP, FPPAG ver the past six months, a number of which slowly releases the drug over time, O significant new drugs have been approved providing up to 13 hours of symptom control. by the Food and Drug Administration (FDA). In The safety and efficacy of the product was addition, several drugs already on the market established in a phase 3 randomized, placebo- have been granted an indication for use in controlled trial in 108 children with ADHD.4 pediatric patients. Following a 5-week open-label dose optimization period, patients were randomized to New Drug Product Approvals treatment (2.5-10 mg) or placebo for a 1-week period. At the end of the week, scores on the Adapalene and Benzoyl Peroxide Swanson, Kotkin, Agler, M-Flynn, and Pelham Epiduo Forte®, a new product containing (SKAMP)-Combined rating scale were adapalene 0.3%, a retinoid, and benzoyl peroxide compared to baseline. The change in scores after 2.5% was approved on July 16, 2015 for the treatment demonstrated a statistically significant treatment of moderate to severe acne in adults improvement throughout the day compared to and children 12 years of age and older.1 The placebo (assessed at 1, 2, 6, 8, 10, 12, and 13 efficacy of the new product was demonstrated in hours post-dose), with a mean change of -8.8 (SE a phase 3 multicenter randomized, double-blind 1.14) in the treated patients and 6.0 (SE 1.19) in trial comparing it to the gel vehicle without the the controls.