BIOGRAPHICAL SKETCH NAME Paul Workman FRS Fmedsci POSITION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fourth Report of Senior Pay and Perks in UK Universities History This
Transparency at the top? The fourth report of senior pay and perks in UK universities History This is the fourth report on pay and perks at the top of British higher education institutions (HEIs) to be published by the University and College Union (UCU). It forms part of the union’s ongoing campaign for greater transparency in higher education, including the rationale behind senior pay rises. UCU submitted a Freedom of Information (FoI) request to 158 HEIs in October 2017. This followed similar requests submitted in 2016, 2015 and 2014. All requests were designed to shine a light on the arbitrary nature of senior pay and perks in universities, and support the union’s call for reform. The basis for this report The FoI request that forms the basis of this report was sent to 158 (HEIs). It requested details of vice-chancellors’ (or head of institution if known by a different title) salaries and those of other senior post-holders earning over £100,000 at the institution during the academic year of 2016/17 (1 August 2016 to 31 July 2017). It also asked for details of flights, spending on hotels, spending on expenses and if the vice-chancellor was provided with accommodation by the university. Finally, we requested to know whether or not the vice-chancellor was a member of the remuneration committee, and requested a copy of the most recently ratified minutes of the institution’s remuneration committee. Variety of responses The questions on expenditure on flights, hotels, expenses and accommodation for vice-chancellors elicited a huge variation in responses with many institutions deploying exemptions under the Freedom of Information Act to avoid providing data. -

Womencount: Leaders in Higher Education 2016
WomenCount Leaders in Higher Education 2016 A report by Norma Jarboe OBE ‘There’s no magic about getting a good gender balance – just dogged repetition of what a high priority it is and a determination to seek out strong women candidates who could hold their own against any competition.’ WomenCount ‘If universities inhibit the progression of talented female staff, they in turn are unable to reach their full potential. We know that universities make a huge contribution to society through research, teaching and partnerships with businesses, among many other activities.’ Professor Dame Athene Donald, Professor of Experimental Physics and Master of Churchill College, University of Cambridge WomenCount are very grateful to Perrett Laver for their support, the Government Equalities Office for their enagagement and Imperial College London for hosting the launch of this report on 2 March 2016. Cover quotation: Sir Nicholas Montagu, Chair of Council, Queen Mary University of London. Published by WomenCount © March 2016, all rights reserved. www.women-count.org Designed and produced by Graffeg. WomenCount: Leaders in Higher Education 2016 Contents 3 Foreword 4 Executive summary 5 Introduction 6 Collective action 9 Collegial governance: diversity challenge or opportunity? 10 Governing bodies: women’s representation on the rise 11 Governing bodies: the balancing act 12 Chairs: few seats for women 14 Vice-Chancellors: barely a fifth are women 15 Chair and Vice-Chancellor teams 16 Executive teams: a pipeline of women leaders 17 Academic heads: less than a third are women 19 HEI income impact women’s leadership 21 Mapping Women’s Leadership in HEIs 22 Reflections on the research 27 The Index 37 Biographies of new Chairs 42 Biographies of new Vice-Chancellors 47 About WomenCount and the author 1 Foreword ‘Gender equality is not a matter of being nice to women. -

Perspective Lecture, Frankfurt February 14Th 2013 Professor Paul
Professor Paul Workman Bio: Perspective Lecture, Frankfurt February 14 th 2013 Professor Paul Workman – Short Biography Professor Paul Workman is a leader in the discovery and development of molecularly targeted cancer drugs and is a passionate advocate of personalized cancer therapy. A molecular pharmacologist and chemical biologist, Paul has been responsible for the discovery and development of a large number of new molecularly targeted cancer drugs. Paul is currently Deputy Chief Executive of The Institute of Cancer Research (ICR) and Director of the ICR’s Cancer Research UK Cancer Therapeutics Unit in Sutton, UK, which is the largest non-profit cancer drug discovery group worldwide. He is also Head of ICR’s Division of Cancer Therapeutics and Harrap Professor of Pharmacology and Therapeutics. Paul obtained his BSc (Hons) in Biochemistry from Leicester University (1973) and his PhD in Cancer Pharmacology from Leeds University (1977). He then moved to Cambridge to become a postdoctoral fellow and scientific staff member of the MRC Clinical Oncology Unit, MRC Centre, Cambridge University (1976-1990) where he established and led the cancer pharmacology group, making major contributions to drugs targeting tumour hypoxia. Following a period as UICC Visiting Fellow at Stanford University and SRI International, California (1989), Paul was appointed as Cancer Research Campaign Professor and Director of Laboratory Research in the Department of Medical Oncology, Beatson Laboratories, Glasgow University (1990-1993). Paul then gained experience in the pharmaceutical industry. From 1993-1997 Paul was Head of the Cancer Research Bioscience Section at AstraZeneca Pharmaceuticals, Alderley Park, UK where he also initiated and led the strategic alliance with Sugen. -

Situación Actual Y Perspectivas De Futuro Personalized Precision Oncology: Current Status and Future Perspectives Madrid, 21 Y 22 De Marzo / March 21-22, 2019
Simposio Internacional International Symposium Oncología personalizada de precisión: situación actual y perspectivas de futuro Personalized Precision Oncology: current status and future perspectives Madrid, 21 y 22 de marzo / March 21-22, 2019 BIO Paul Workman Professor Paul Workman FMedSci, FRS is Chief Executive and President of The Institute of Cancer Research (ICR). Professor Workman is a passionate advocate of personalised molecular medicine and is an enthusiastic practitioner of multidisciplinary cancer drug discovery and development approaches to 'drugging the cancer genome'. He also conceptualised the 'Pharmacological Audit Trail' approach. As well as establishing and successfully leading drug discovery project teams yielding clinical candidates, Professor Workman’s personal and collaborative research utilises molecular pharmacology and chemical biology approaches, including high-throughput and genome-wide as well as hypothesis- driven strategies, to interrogate cancer biology, identify and validate new drug targets, discover and develop chemical tools and drugs acting on these targets, identify predictive and mechanism of action biomarkers, and elucidate mechanisms of drug sensitivity of resistance. He is especially interested in exploiting the addictions, vulnerabilities and dependencies of cancer cells using a combination of small molecule tools and drugs alongside molecular genetic techniques. Professor Workman has successfully built a series of multidisciplinary drug discovery and development teams in the academic, large pharma and biotechnology company sectors. Through this experience he has been able to combine the best elements of each of these environments. He has been responsible for the discovery of a number of drug development candidates, including in particular pathfinding inhibitors of the HSP90 molecular chaperone and the PI3 kinase family of signalling enzymes. -
BSCB Newsletter 2019A:BSCB Aut2k7
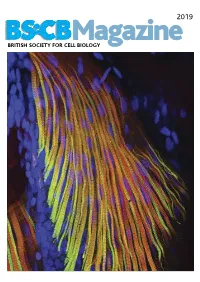
2019 BSCB Magazine BRITISH SOCIETY FOR CELL BIOLOGY 2019 CONTENTS BSCB Magazine News 2 Book reviews 8 Features 9 Meeting Reports 21 Summer students 25 Society Business 32 Editorial Front cover: microscopic Welcome to the 2019 BSCB Magazine! This year Mustafa Aydogan (University of Oxford), as well as to structure of pectoral fin and Susana and Stephen are filling in for our Newsletter BSCB postdoc poster of the year winners Dr Anna hypaxial muscles of a zebrafish Editor Ann Wheeler. We hope you will enjoy this Caballe (University of Oxford) and Dr Agata Gluszek- Danio rerio larvae at four days year’s magazine! Kustusz (University of Edinburgh). post fertilization. The immunostaining highlights the This year we had a number of fantastic one day In 2019, we will have our jointly BSCB-BSDB organization of fast (red) and meetings sponsored by BSCB. These focus meetings Spring meeting at Warwick University from 7th–10th slow (green) myosins. All nuclei are great way to meet and discuss your science with April, organised by BSCB members Susana Godinho are highlighted in blue (hoechst). experts in your field and to strengthen your network of and Vicky Sanz-Moreno. The programme for this collaborators within the UK. You can read more about meeting, which usually provides a broad spectrum of these meetings in the magazine. If you have an idea themes, has a focus on cancer biology: cell for a focus one day meeting, check how to apply for migration/invasion, organelle biogenesis, trafficking, funding on page 4. Our ambassadors have also been cell-cell communication. -

CICR Newsletter Template
1. From the Editors From the Editors, with Dr Iain D.G. Watson, Editorial Board Member Each quarter, the editorial board selects an area to highlight from the broad range of topics that fall under the umbrella of chemistry in cancer research. Our topic this quarter is the ubiquitin proteasome system. CICR editorial board member Iain Watson has taken the lead in assembling an overview of the topic. The ubiquitin proteasome system (UPS) In 2004 the Nobel Prize in Chemistry was awarded to Aaron Ciechanover, Avram Hershko and Irwin Rose for the discovery of ubiquitin-mediated proteolysis. These are the cellular processes by which proteins are identified for breakdown and elimination. Central to this system is the post-translational tagging of proteins with ubiquitin, a 76 amino-acid protein. Ubiquitin is activated, conjugated and ligated onto target proteins, most commonly between a lysine on the target protein and the C-terminal glycine of ubiquitin. This collaborative cascade is catalyzed by enzymes known as E1, E2 and E3 ligases, where an E1 enzyme may bind many E2s, which bind many E3s hierarchically, allowing regulation of the cellular machinery. Ubiquitination is reversible which is catalyzed by families of deubiquitinase enzymes (DUBs). Tagged proteins are transported to a large cylindrical multisubunit protease complex called the proteasome to be degraded. The common 26S proteasome is comprised of one 20S core particle and two regulatory 19S particles which recognize the ubiquitinated proteins, unfold them and feed them into the catalytic core. Numerous cellular processes are regulated by ubiquitin-mediated proteolysis including the cell cycle, DNA repair, transcription, protein quality control and the immune response. -

Year in Review
Year in review For the year ended 31 March 2017 Trustees2 Executive Director YEAR IN REVIEW The Trustees of the Society are the members Dr Julie Maxton of its Council, who are elected by and from Registered address the Fellowship. Council is chaired by the 6 – 9 Carlton House Terrace President of the Society. During 2016/17, London SW1Y 5AG the members of Council were as follows: royalsociety.org President Sir Venki Ramakrishnan Registered Charity Number 207043 Treasurer Professor Anthony Cheetham The Royal Society’s Trustees’ report and Physical Secretary financial statements for the year ended Professor Alexander Halliday 31 March 2017 can be found at: Foreign Secretary royalsociety.org/about-us/funding- Professor Richard Catlow** finances/financial-statements Sir Martyn Poliakoff* Biological Secretary Sir John Skehel Members of Council Professor Gillian Bates** Professor Jean Beggs** Professor Andrea Brand* Sir Keith Burnett Professor Eleanor Campbell** Professor Michael Cates* Professor George Efstathiou Professor Brian Foster Professor Russell Foster** Professor Uta Frith Professor Joanna Haigh Dame Wendy Hall* Dr Hermann Hauser Professor Angela McLean* Dame Georgina Mace* Dame Bridget Ogilvie** Dame Carol Robinson** Dame Nancy Rothwell* Professor Stephen Sparks Professor Ian Stewart Dame Janet Thornton Professor Cheryll Tickle Sir Richard Treisman Professor Simon White * Retired 30 November 2016 ** Appointed 30 November 2016 Cover image Dancing with stars by Imre Potyó, Hungary, capturing the courtship dance of the Danube mayfly (Ephoron virgo). YEAR IN REVIEW 3 Contents President’s foreword .................................. 4 Executive Director’s report .............................. 5 Year in review ...................................... 6 Promoting science and its benefits ...................... 7 Recognising excellence in science ......................21 Supporting outstanding science ..................... -

LONG-TERM MEMBERS 25+ Years of Membership
LONG-TERM MEMBERS 25+ Years of Membership Stuart A. Aaronson, MD Stephen P. Ackland, MBBS Carol Aghajanian, MD Steven A. Akman, MD Icahn School of Medicine at Mount Sinai University of Newcastle Memorial Sloan Kettering Cancer Center Roper St. Francis Healthcare United States Australia United States United States Active Member Active Member Active Member Active Member 38 Years of Membership 33 Years of Membership 27 Years of Membership 35 Years of Membership Cory Abate-Shen, PhD Edward M. Acton, PhD Irina U. Agoulnik, PhD Emmanuel T. Akporiaye, PhD Columbia University Irving Medical United States Florida International University Verana Therapeutics Center Emeritus Member United States United States United States 42 Years of Membership Active Member Emeritus Member Active Member 25 Years of Membership 31 Years of Membership 26 Years of Membership David J. Adams, PhD Duke University Medical Center Imran Ahmad, PhD Ala-Eddin Al Moustafa, PhD James L. Abbruzzese, MD United States Northwestern Medicine McGill University Duke University Emeritus Member United States Canada United States 32 Years of Membership Active Member Active Member Active Member 25 Years of Membership 26 Years of Membership 32 Years of Membership Gregory P. Adams, PhD Elucida Oncology Nihal Ahmad, PhD Abdul Al Saadi, PhD Ehtesham A. Abdi, MBBS United States Univ. of Wisconsin Madison Sch. of Med. William Beaumont Hospital The Tweed Hospital Active Member & Public Health United States Australia 29 Years of Membership United States Emeritus Member Emeritus Member Active Member 52 Years of Membership 33 Years of Membership Lucile L. Adams-Campbell, PhD 25 Years of Membership Georgetown Lombardi Comprehensive Suresh K. -

Biographical Sketch Format Page
BIOGRAPHICAL SKETCH NAME POSITION TITLE Professor Paul Workman FRS FMedSci 1. Chief Executive and President, The Institute of Cancer Research (ICR), London UK 2. Director, CRUK-ICR Centre in association with The Royal Marsden NHS Trust, London UK 3. Harrap Professor of Pharmacology and Therapeutics, University of London, UK EDUCATION/TRAINING DEGREE INSTITUTION AND LOCATION (if YY FIELD OF STUDY applicable) University of Leicester, UK BSc (Hons) 1973 Biological Sciences University of Leeds, UK PhD 1977 Cancer Pharmacology A. Personal Statement I am an experienced cancer research scientist with extensive leadership and administrative experience in academia and industry. I am passionate about translating our rapidly emerging understanding of fundamental cancer biology with a sense of urgency into new personalized medicines for cancer patients. I am currently Chief Executive and President of The Institute of Cancer Research (ICR), London and Harrap Professor of Pharmacology and Therapeutics at ICR – a college of the University of London. In addition, I am Director of the CRUK-ICR Centre in association with The Royal Marsden NHS Trust (RM). Furthermore, I am the inaugural Director of the ICR-Imperial College Cancer Research Centre of Excellence in Cancer Research which was established in 2016 to bring together – in synergistic convergence science – Imperial’s strengths in engineering, physical, natural, and data sciences and medicine with ICR’s strengths in basic and applied cancer research, as well as forming the basis for an application to become a CRUK Major Centre, addressing all aspects of cancer prevention, early detection and treatment. ICR came top in REF2014 and ICR/RM has been ranked as one of the top four cancer centres in the world for the impact of its publications (Thomson Reuters Evidence). -

3Rd EFMC Young Medicinal Chemist Symposium
3rd EFMC Young Medicinal Chemist Symposium September 1-2, 2016 | Manchester | United Kingdom Book of Abstracts Content Welcome 2 Sponsors 3 Exhibitors’ Company Profile 5 Programme 6 Flash Poster Presentations List 9 Keynote Lectures 11 Winners of the National Medicinal Chemist Meeting 15 Oral Communications 31 Flash Poster Presentations 37 Posters Abstracts 59 List of abstracts 119 List of participants 125 1 Welcome Dear participant On behalf of the European Federation for Medicinal Chemistry (EFMC) and the Organizing Committee we warmly welcome you to Manchester for the 3rd edition of the EFMC Young Medicinal Chemist Symposium (EFMC-YMCS). This edition has been organised on behalf of the EFMC in connection with the XXIV EFMC International Symposium on Medicinal Chemistry (EFMC-ISMC 2016) in keeping with the initial aim of: • Creating a network of young European investigators in Medicinal Chemistry • Stimulating young European investigators in Medicinal Chemistry to share their scientific work with peers and inspiring leaders in the field • Creating competition and excellence in Medicinal Chemistry within Europe by selecting the European Champion in Medicinal Chemistry Over 125 scientists coming from 30 nations will gather at the Manchester Institute of Biotechnology for this exciting mini-symposium. The 2 keynote lectures, 14 oral communications by invited prize winners from national young medicinal chemist meetings in Europe, 4 additional selected oral communications, 20 Flash Poster Presentations and more than 70 poster presentations -

Improving Recognition of Team Science Contributions in Biomedical Research Careers
Improving recognition of team science contributions in biomedical research careers March 2016 The Academy of Medical Sciences is most grateful to Professor Anne Ridley FMedSci and to the members of the Working Group for undertaking this project. We thank the Academy’s Council members and staff, external review group, workshop attendees and all individuals who contributed to the report. This report is published by the Academy and has been endorsed by its Officers and Council. Contributions by the Working Group were made purely in an advisory capacity. The members of the Working Group participated in an individual capacity and not as representatives of, or on behalf of, their affiliated hospitals, universities, organisations or associations. All web references were accessed in November 2015. This work is © The Academy of Medical Sciences and is licensed under Creative Commons Attribution 4.0 International. Improving recognition of team science contributions in biomedical research careers Contents Executive summary ..................................................................................................... 4 Recommendations ...................................................................................................... 6 Glossary of terms and abbreviations .......................................................................... 10 1. Introduction ...........................................................................................................12 2. Method of working .............................................................................................. -

Final Program
The 9th Annual Conference of the International Chemical Biology Society ICBS2020VIRTUAL Addressing Therapeutic Challenges Together with Chemical Biology November 11, 2020 7:30 PM to 11:30 PM EST November 12, 2020 9:30 AM to 2:00 PM EST November 13, 2020 9:30 AM to 2:40 PM EST 9th Annual Conference | November 11-13, 2020 | Virtual Table of Contents Table of Contents ............................................................ 1 Session 3: Academic-Industry Partnerships Acknowledgements ........................................................ 1 case studies .................................................................... 23 ICBS Board of Directors ................................................ 2 Session 4: Chemical Biology of Small Molecule About ICBS ...................................................................... 2 Protein Degraders ........................................................ 28 ICBS International Advisory Board ............................. 3 EFMC Speaker .............................................................. 32 ICBS Global Council ...................................................... 4 Session 5: Chemical Technologies to Promote Welcome Letter ............................................................... 5 Drug Discovery ............................................................. 33 Trainee Pre-Conference Program ................................ 6 Rising Stars Session ...................................................... 37 Detailed Program ..........................................................