5Th Annual Meeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fourth Report of Senior Pay and Perks in UK Universities History This
Transparency at the top? The fourth report of senior pay and perks in UK universities History This is the fourth report on pay and perks at the top of British higher education institutions (HEIs) to be published by the University and College Union (UCU). It forms part of the union’s ongoing campaign for greater transparency in higher education, including the rationale behind senior pay rises. UCU submitted a Freedom of Information (FoI) request to 158 HEIs in October 2017. This followed similar requests submitted in 2016, 2015 and 2014. All requests were designed to shine a light on the arbitrary nature of senior pay and perks in universities, and support the union’s call for reform. The basis for this report The FoI request that forms the basis of this report was sent to 158 (HEIs). It requested details of vice-chancellors’ (or head of institution if known by a different title) salaries and those of other senior post-holders earning over £100,000 at the institution during the academic year of 2016/17 (1 August 2016 to 31 July 2017). It also asked for details of flights, spending on hotels, spending on expenses and if the vice-chancellor was provided with accommodation by the university. Finally, we requested to know whether or not the vice-chancellor was a member of the remuneration committee, and requested a copy of the most recently ratified minutes of the institution’s remuneration committee. Variety of responses The questions on expenditure on flights, hotels, expenses and accommodation for vice-chancellors elicited a huge variation in responses with many institutions deploying exemptions under the Freedom of Information Act to avoid providing data. -

Guide to Publication Policies of the Nature Journals
GUIDE TO PUBLICATION POLICIES OF THE NATURE JOURNALS Last updated on 30 April 2009. Editorial Policies NATURE JOURNALS' POLICIES ON PUBLICATION ETHICS Nature journals' authorship policy Being an author The Nature journals do not require all authors of a research paper to sign the letter of submission, nor do they impose an order on the list of authors. Submission to a Nature journal is taken by the journal to mean that all the listed authors have agreed all of the contents. The corresponding (submitting) author is responsible for having ensured that this agreement has been reached, and for managing all communication between the journal and all co-authors, before and after publication. Any changes to the author list after submission, such as a change in the order of the authors, or the deletion or addition of authors, needs to be approved by a signed letter from every author. Responsibilities of senior team members on multi-group collaborations The editors at the Nature journals assume that at least one member of each collaboration, usually the most senior member of each submitting group or team, has accepted responsibility for the contributions to the manuscript from that team. This responsibility includes, but is not limited to: (1) ensuring that original data upon which the submission is based is preserved and retrievable for reanalysis; (2) approving data presentation as representative of the original data; and (3) foreseeing and minimizing obstacles to the sharing of data, materials, algorithms or reagents described in the work. Author contributions statementsAuthors are required to include a statement of responsibility in the manuscript that specifies the contribution of every author. -

Pedersen, Steen Bønnelykke; Christensen, Lars Porskjær
Syddansk Universitet Screening of plant extracts for anti-inflammatory activity Radko, Yulia ; Pedersen, Steen Bønnelykke; Christensen, Lars Porskjær Publication date: 2015 Document version Final published version Citation for pulished version (APA): Radko, Y., Pedersen, S. B., & Christensen, L. P. (2015). Screening of plant extracts for anti-inflammatory activity. Abstract from Annual Meeting of the American Society of Pharmacognosy, Copper Mountain, CO, United States. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 19. Apr. 2017 1 2015 Annual Meeting of the ASP | July 25th–29th, 2015 | Copper Mountain, CO, USA 2 Dear Fellow Natural Product Enthusiasts, “Natural Products Rising to the Top,” was selected as the theme for the 2015 American Society of Pharmacognosy (ASP) Meeting. This topic symbolizes both the fact that this year’s meeting will take place at the highest altitude of any ASP meet- ing held to date, as well as the profound rise in interests in natural products across many disciplines. -

The Nature Index Journals
The Nature Index journals The current 12-month window on natureindex.com includes data from 57,681 primary research articles from the following science journals: Advanced Materials (1028 articles) American Journal of Human Genetics (173 articles) Analytical Chemistry (1633 articles) Angewandte Chemie International Edition (2709 articles) Applied Physics Letters (3609 articles) Astronomy & Astrophysics (1780 articles) Cancer Cell (109 articles) Cell (380 articles) Cell Host & Microbe (95 articles) Cell Metabolism (137 articles) Cell Stem Cell (100 articles) Chemical Communications (4389 articles) Chemical Science (995 articles) Current Biology (440 articles) Developmental Cell (204 articles) Earth and Planetary Science Letters (608 articles) Ecology (259 articles) Ecology Letters (120 articles) European Physical Journal C (588 articles) Genes & Development (193 articles) Genome Research (184 articles) Geology (270 articles) Immunity (159 articles) Inorganic Chemistry (1345 articles) Journal of Biological Chemistry (2639 articles) Journal of Cell Biology (229 articles) Journal of Clinical Investigation (298 articles) Journal of Geophysical Research: Atmospheres (829 articles) Journal of Geophysical Research: Oceans (493 articles) Journal of Geophysical Research: Solid Earth (520 articles) Journal of High Energy Physics (2142 articles) Journal of Neuroscience (1337 articles) Journal of the American Chemical Society (2384 articles) Molecular Cell (302 articles) Monthly Notices of the Royal Astronomical Society (2946 articles) Nano Letters -

Enthusing Children About Chemistry Climate Change and Biogenic Emissions Predicting Properties Using Informatics the Oil Industr
Summer 2008 Enthusing children about chemistry Predicting properties using informatics Climate change and biogenic emissions The oil industry’s chemical challenges As I see it... So are you finding it increasingly diffi - Oil exploration doesn’t just offer a career for engineers – cult to attract good chemists? chemists are vital, too. Sarah Houlton spoke to Schlumberger’s It can be a challenge, yes. Many of the com - pany’s chemists are recruited here, and they Tim Jones about the crucial role of chemistry in the industry often move on to other sites such as Houston or Paris, but finding them in the first place can be a challenge. Maybe one reason is that the oil People don’t think of Schlumberger as We can’t rely on being able to find suitable industry doesn’t have the greatest profile in a chemistry-using company, but an chemistries in other industries, either, mainly chemistry, and people think it employs engi - engineering one. How important is because of the high temperatures and pressures neers, not chemists. But it’s something the chemistry in oil exploration? that we have to be able to work at. Typically, the upstream oil industry cannot manage without, It’s essential! There are many challenges for upper temperature norm is now 175°C, but even if they don’t realise it! For me, maintaining chemistry in helping to maintain or increase oil we’re increasingly looking to go over 200°C. – if not enhancing – our recruitment is perhaps production. It’s going to become increasingly For heavy oil, where we heat the oil up with one of the biggest issues we face. -

Womencount: Leaders in Higher Education 2016
WomenCount Leaders in Higher Education 2016 A report by Norma Jarboe OBE ‘There’s no magic about getting a good gender balance – just dogged repetition of what a high priority it is and a determination to seek out strong women candidates who could hold their own against any competition.’ WomenCount ‘If universities inhibit the progression of talented female staff, they in turn are unable to reach their full potential. We know that universities make a huge contribution to society through research, teaching and partnerships with businesses, among many other activities.’ Professor Dame Athene Donald, Professor of Experimental Physics and Master of Churchill College, University of Cambridge WomenCount are very grateful to Perrett Laver for their support, the Government Equalities Office for their enagagement and Imperial College London for hosting the launch of this report on 2 March 2016. Cover quotation: Sir Nicholas Montagu, Chair of Council, Queen Mary University of London. Published by WomenCount © March 2016, all rights reserved. www.women-count.org Designed and produced by Graffeg. WomenCount: Leaders in Higher Education 2016 Contents 3 Foreword 4 Executive summary 5 Introduction 6 Collective action 9 Collegial governance: diversity challenge or opportunity? 10 Governing bodies: women’s representation on the rise 11 Governing bodies: the balancing act 12 Chairs: few seats for women 14 Vice-Chancellors: barely a fifth are women 15 Chair and Vice-Chancellor teams 16 Executive teams: a pipeline of women leaders 17 Academic heads: less than a third are women 19 HEI income impact women’s leadership 21 Mapping Women’s Leadership in HEIs 22 Reflections on the research 27 The Index 37 Biographies of new Chairs 42 Biographies of new Vice-Chancellors 47 About WomenCount and the author 1 Foreword ‘Gender equality is not a matter of being nice to women. -

Suzanne Pfeffer
July 2010 ASBMB PreSidentiAl PriMer: Suzanne Pfeffer American Society for Biochemistry and Molecular Biology AAdjuvdjuvAAntnt IImmunothermmunotherAApypy ususIIngng KKrnrn70007000 KRN7000 (α-Galactosyl Ceramide) Avanti Number 867000 Supplier: Funakoshi Co. Ltd. Hepatic metastasis is a major clinical problem in cancer treatment. We examined antitumor ac- tivity of alpha-galactosylceramide (KRN7000) on mice with spontaneous liver metastases of re- ticulum cell sarcoma M5076 tumor cells (spontaneous metastasis model). In this model, all mice that were s.c. challenged with one million tumor cells developed a solid s.c. mass by day 7 and died of hepatic metastases. In the current study, we administered 100 microg/kg of KRN7000 to the model mice on days 7, 11, and 15. This treatment suppressed the growth of established liver metastases and resulted in the prolongation of survival time. Fluorescence-activated cell sorter analysis of phenotypes of spleen cells, hepatic lymphocytes, and regional lymph node cells around the s.c. tumor revealed that CD3+NK1.1+ (NKT) cells increased in hepatic lym- phocytes of the KRN7000-treated mice. Cytotoxic activity and IFN-gamma production of hepatic lymphocytes were augmented in comparison with those of spleen cells and regional LN cells. At the same time, interleukin (IL)-12 production of hepatic lymphocytes was markedly enhanced. Neutralization of IL-12 using a blocking monoclonal antibody diminished the prolonged survival time. These results showed that the in vivo antitumor effects of KRN7000 on spontaneous liver metastases were dependent on the endogenous IL-12 production, where NKT cells in the liver are suggested to be involved. Adjuvant immunotherapy using KRN7000 could be a promising modality for the prevention of postoperative liver metastases. -

NIH Conflict of Interest Regs Revised
OCTOBEROCTOBER 2005 www.asbmb.org Constituent Society of FASEB AMERICAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY NIH Conflict of Interest Regs Revised SEE PAGE 30 FOR NEW CLARA BENSON TRAVEL FELLOWSHIP AWARD Held in conjunction with EB2006 Custom Antibodies Your Way! Choose the protocol that is right for you! QwikScreen ™: 65 day, 2 rabbit protocol - 4 immunizations, 3 bleeds/rabbit (~100ml serum), customer supplied peptide/protein - Options: Peptide synthesis, immunograde Conjugation to carrier u ELISA u u Animal extensionsMS analysis $685 Standard: 80 day, 2 rabbit protocol - 5 immunizations, 5 bleeds/rabbit (~ 200ml ser Options: um), ELISA, customer supplied peptide/pr Peptide synthesis MS Check™ peptide sequence confirmation u HPLC purified peptide Affinity purification otein - Pinnacle: $975 u HPLC and MS analysis u Complete Affinity Purified Protocol- Animal extensions 2 rabbit pr 5 bleeds/rabbitotocol, (~ 200mlepitope serum), design, peptide PhD technical synthesis support, (up to 20mer),5 immunizations, HPLC purified to ~85%, 5+mg peptide to customer, ELISA, evaluation period, affinity purification, and morMS Check™ peptide sequence confirmationNo Hidden Charges! e… - Discounts for Multiple Protocols$1795 , Includes peptide sequencing by CID MS/MS– u Guaranteed Peptide Let our enthusiasm for scienceExpert workTechnical for SupportFidelity! P: 508.303.8222 www.21stcenturybio.com Toll-free: 877.217.8238 F: 508.303.8333 you! E: [email protected] www.asbmb.org AMERICAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY OCTOBER -
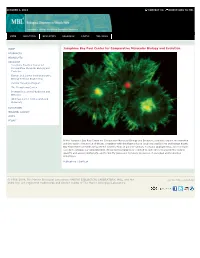
Josephine Bay Paul Center for Comparative Molecular Biology And
OCTOBER 2, 2014 CONTACT US DIRECTIONS TO MBL HOME ABOUT MBL EDUCATION RESEARCH GIVING MBL NEWS HOME Josephine Bay Paul Center for Comparative Molecular Biology and Evolution RESOURCES HIGHLIGHTS RESEARCH Josephine Bay Paul Center for Comparative Molecular Biology and Evolution Eugene Bell Center for Regenerative Biology & Tissue Engineering Cellular Dynamics Program The Ecosystems Center Program in Sensory Physiology and Behavior Whitman Center for Research and Discovery EDUCATION MBLWHOI LIBRARY GIFTS PEOPLE In the Josephine Bay Paul Center for Comparative Molecular Biology and Evolution, scientists explore the evolution and interaction of genomes of diverse organisms with significant roles in environmental biology and human health. Bay Paul Center scientists integrate the powerful tools of genome science, molecular phylogenetics, and molecular ecology to advance our understanding of how living organisms are related to each other, to provide the tools to quantify and assess biodiversity, and to identify genes and metabolic processes of ecological and biomedical importance. Publications | Staff List © 1996-2014, The Marine Biological Laboratory MARINE BIOLOGICAL LABORATORY, MBL, and the Join the MBL community: 1888 logo are registered trademarks and service marks of The Marine Biological Laboratory. OCTOBER 2, 2014 CONTACT US DIRECTIONS TO MBL HOME ABOUT MBL EDUCATION RESEARCH GIVING MBL NEWS HOME Bay Paul Center Publications RESOURCES Akerman, NH; Butterfield, DA; and Huber, JA. 2013. Phylogenetic Diversity and Functional Gene Patterns of Sulfur- oxidizing Subseafloor Epsilonproteobacteria in Diffuse Hydrothermal Vent Fluids. Front Microbiol. 4, 185. HIGHLIGHTS RESEARCH Alliegro, MC; and Alliegro, MA. 2013. Localization of rRNA Transcribed Spacer Domains in the Nucleolinus and Maternal Procentrosomes of Surf Clam (Spisula) Oocytes.” RNA Biol. -

Genetically Encoded Fragment-Based Discovery
Available online at www.sciencedirect.com ScienceDirect Genetically encoded fragment-based discovery 1 Ratmir Derda and Simon Ng This opinion describes recent advances of molecular discovery the fragment of interest. This opinion focuses on chemi- technology dubbed Genetically Encoded Fragment-Based cal post-translational incorporation of fragments Discovery (GE-FBD). GE-FBD starts from a known ligand or (Figure 1b). Alternatively, {FP} libraries can be built from ‘fragment’ that binds to a desired target weakly and often with unnatural amino acids (UAA) that contain fragments as low specificity. Covalent incorporation of fragment into a part of their side-chain (Figure 1c). Selection from {FP} diverse, genetically encoded library of peptides yields a library libraries has been shown to identify ligands in which the of peptide–fragment combinations. Selection from such a fragment covalently linked to peptide segment bind the library has a high likelihood to identify ligands, in which the target with improved affinity and specificity when com- peptides bind to distinct adjacent pockets of the target in pared to the original fragment (Figure 1d). Improvement F FP synergy with the fragment and exhibits enhanced affinity and can be defined as r = KD/ KD > 1 or DDGbind Àlog F specificity when compared to the fragment itself. GE-FBD (r) < 0, where KD is the affinity of the fragment towards could employ fragments that bind non-covalently as well as the target of interest (Figure 1e). Figure 1d summarizes reversible covalent warheads. The key advances in GE-FBD up-to-date outcomes from published GE-FBD reports include (i) synthetic chemistry that enables incorporation of and shows that r = 10–100 can be found across diverse F diverse fragments into both linear and cyclic peptide libraries; fragment with KD ranging from 100 nM to 1 mM [3 ,4– (ii) quantification of multi-step modifications in million-to-billion 6,7 ,8,9 ,10,11 ,12,13 ,14]. -
![Ecosystems for Innovation [Compatibility Mode]](https://docslib.b-cdn.net/cover/4930/ecosystems-for-innovation-compatibility-mode-1254930.webp)
Ecosystems for Innovation [Compatibility Mode]
Ecosystems for innovation Claire Ruskin CEO, Cambridge Network Innovation in Cambridge – how did it happen, how can we grow it and is it repeatable elsewhere? Material from Shai Vyakarnum – Judge Business School Tim Minshall – Institute for Manufacturing Claire Ruskin – CEO Cambridge Network Steven Ireland – East of England Inward Investment Cambridge Network – II EE www.cambridgenetwork.co.uk What’s special about Cambridge? The starting point … • A global ‘top five’ university: The University of Cambridge has 800 years at the top • Proximity to London and Europe : 5 international airports within 2 hours • Highly qualified employees: > 40% of people living in Cambridge having a high level qualification (compared to the national average of < 20%) • A few hi-tech companies back in the fifties • The start of a world class contract R&D cluster (the consultancies) from 1960 • => Evolving to a hi-tech cluster supporting > 143,000 jobs in the region. The cluster generates the equivalent of an NPV of £53bn in GDP. • => Good quality of life: Polls highlight Cambridgeshire as one of the best places to live in the UK • => Attitude: a good feeling about success and starting something new Cambridge Network – II EE www.cambridgenetwork.co.uk Why will Cambridge continue to have competitive advantage? • Diverse science base and research infrastructure, bringing excellent people to the Universities, business and medical organisations • Practice at innovation on demand as well as commercialisation • Collective learning and networking systems • Entrepreneurial -

CURRICULUM VITAE Robert Michael Williams, Ph.D
CURRICULUM VITAE Robert Michael Williams, Ph.D. University Distinguished Professor of Chemistry Department of Chemistry, Colorado State University, Fort Collins, CO 80523 Phone (970) 491-6747; FAX (970)-491-3944 e-mail: [email protected] Webpage: http://rwindigo1.chm.colostate.edu/ Personal Information: Date of birth: February 8, 1953 Married: Jill Janssen Williams Children: Ridge Janssen Williams (born February 23, 2001) Rainier Valentine Williams (born August 20, 2005) Education: B.A., Chemistry (with highest distinction), May, 1975. Syracuse University, Syracuse, NY. Thesis under Professor Ei-ichi Negishi on "A Stereoselective Synthesis of Partially Substituted 1,2,3-Butatriene Derivatives via Hydroboration". Ph.D., Organic Chemistry, June, 1979. Massachusetts Institute of Technology, Cambridge, MA. Thesis advisor, Dr. W. H. Rastetter. Thesis title: "Epidithiapiperazinedione Syntheses". Postdoctoral Fellow, September 1979-September 1980, Harvard University, Cambridge, MA. The late Professor R. B. Woodward group (Y. Kishi, principal investigator). Total synthesis of erythromycin A. Honors and Awards: Organic Synthesis Award, Local Rocky Mountain ACS Section, Reaching New Heights (2012) JSPS Invitation Fellowship Program for Research in Japan (Long-Term) 2012-2013 Ernest Guenther Award in the Chemistry of Natural Products, American Chemical Society (2011) Multiple Myeloma Research Foundation Senior Award (2009-2011) University Distinguished Professor, Colorado State University (2002) Arthur C. Cope Scholar Award, American