Diversity of Root-Associated Culturable Fungi of Cephalanthera Rubra (Orchidaceae) in Relation to Soil Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

November December 2010 Vol 67.3 Victoria Natural
NOVEMBER DECEMBER 2010 VOL 67.3 VICTORIA NATURAL HISTORY SOCIETY The Victoria Naturalist Vol. 67.3 (2010) 1 Published six times a year by the SUBMISSIONS VICTORIA NATURAL HISTORY SOCIETY, P.O. Box 5220, Station B, Victoria, BC V8R 6N4 Deadline for next issue: December 1, 2010 Contents © 2010 as credited. Send to: Claudia Copley ISSN 0049—612X Printed in Canada 657 Beaver Lake Road, Victoria BC V8Z 5N9 Editors: Claudia Copley, 250-479-6622, Penelope Edwards Phone: 250-479-6622 Desktop Publishing: Frances Hunter, 250-479-1956 e-mail: [email protected] Distribution: Tom Gillespie, Phyllis Henderson, Morwyn Marshall Printing: Fotoprint, 250-382-8218 Guidelines for Submissions Opinions expressed by contributors to The Victoria Naturalist Members are encouraged to submit articles, field trip reports, natural are not necessarily those of the Society. history notes, and book reviews with photographs or illustrations if possible. Photographs of natural history are appreciated along with VICTORIA NATURAL HISTORY SOCIETY documentation of location, species names and a date. Please label your Honorary Life Members Dr. Bill Austin, Mrs. Lyndis Davis, submission with your name, address, and phone number and provide a Mr. Tony Embleton, Mr. Tom Gillespie, Mrs. Peggy Goodwill, title. We request submission of typed, double-spaced copy in an IBM compatible word processing file on diskette, or by e-mail. Photos and Mr. David Stirling, Mr. Bruce Whittington slides, and diskettes submitted will be returned if a stamped, self- Officers: 2009-2010 addressed envelope is included with the material. Digital images are PRESIDENT: Darren Copley, 250-479-6622, [email protected] welcome, but they need to be high resolution: a minimum of 1200 x VICE-PRESIDENT: James Miskelly, 250-477-0490, [email protected] 1550 pixels, or 300 dpi at the size of photos in the magazine. -

Physiological Features of the Terrestrial Orchids
Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies)Voll. XLIX/2019 PHYSIOLOGICAL FEATURES OF THE TERRESTRIAL ORCHIDS CEPHALANTHERA LONGIFOLIA AND PLATANTHERA BIFOLIA THAT GROW IN THE PEDO-CLIMATIC CONDITIONS FROM OLTENIA REGION OF ROMANIA BUSE-DRAGOMIR LUMINITA (1), NICOLAE ION (2), NICULESCU MARIANA (3) (1) University of Craiova, E-mail: [email protected] (2) University of Craiova, E-mail: [email protected] (3) University of Craiova, E-mail: [email protected] *Corresponding author email: [email protected] Key words: terrestrial orchids, transpiration, photosynthesis, light ABSTRACT For studying the physiology of terrestrial orchids, plants from the species Cephalanthera longifolia and Platanthera bifolia have been used. The experiences were carried out directly on the field, in Mehedinţi County, Comanesti Hills. In both species of orchids, the seasonal dynamics of photosynthesis registers a peak during the flowering period that corresponds to a maximum content of assimilating pigments and also a maximum leaf surface. In terrestrial orchids, the seasonal dynamics of leaf transpiration intensity is highest during spring, when the water content in the soil is high and minimal in summer. The graphs showing the diurnal variation of photosynthesis for the two species indicate that Cephalanthera longifolia prefers semi-shaded and sunny habitats, while the plants of Platanthera bifolia that are found in the studied areas prefer a more shaded environment INTRODUCTION Orchids that grow directly at the two, more or less globular or digitally- ground level in large areas of Europe or branched tubules. From the main tuber on the grasslands of the tropical regions comes the flowering stem. -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -
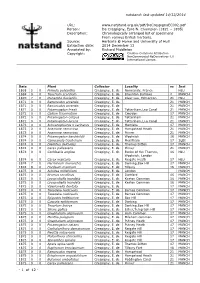
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Watsonia 14 (1982), 79-100
Walsonia , 14, 79-100 (\982) 79 Book Reviews Flowers of Greece and the Balkans. A field guide. Oleg Polunin. Pp. xv+592 including 62 pages of line drawings and 21 maps, with 80 colour plates. Oxford University Press, Oxford. 1980. Price £40.00 (ISBN 0-19- 217-6269). This latest of Oleg Polunin's Field guides to the European flora presents a concise, but by no means superficial, picture of the flowering plants and conifers of the Balkan peninsula. The area that is covers takes in the whole of Greece (including the East Aegean Islands, excluded from Flora Europaea), Turkey-in-Europe, Albania, Bulgaria, Jugoslavia as far north as the River Sava, and the small portion of Romania that lies south and east of the River Danube. The author has condensed his account of the rich and varied flora of the region, together with the associated mass of published material, into a form that is attractive, comprehensible and useful to the amateur botanist. A book of this type has been badly needed, as the available floristic texts on the Balkans tend to be over 50 years old, scarce, extremely expensive and written in a foreign language, often Latin. However, the most significant attribute of this book is not that it gives access to diffuse and obscure information, but that it provides a radical alternative to popular botanical accounts of Greece which emphasize the lowland spring flora, notably the orchids and other petaloid monocots. Based on the author's many years of botanical experience and extensive travel in the region, Flowers of Greece and the Balkans demonstrates the wide range of flora and vegetation that the amateur botanist can expect to see in the Balkan peninsula. -

Morphology and Molecules: the Sebacinales, a Case Study
Mycol Progress DOI 10.1007/s11557-014-0983-1 ORIGINAL ARTICLE Morphology and molecules: the Sebacinales, a case study Franz Oberwinkler & Kai Riess & Robert Bauer & Sigisfredo Garnica Received: 4 April 2014 /Accepted: 8 April 2014 # German Mycological Society and Springer-Verlag Berlin Heidelberg 2014 Abstract Morphological and molecular discrepancies in the irregular germinating spores and inconspicuous cystidia, and biodiversity of monophyletic groups are challenging. The S. flagelliformis with flagelliform dikaryophyses from intention of this study was to find out whether the high S. epigaea s.str. Additional clades in Sebacina, based on molecular diversity in Sebacinales can be verified by micro- molecular differences, cannot be distinguished morphologi- morphological characteristics. Therefore, we carried out mo- cally at present. lecular and morphological studies on all generic type species of Sebacinales and additional representative taxa. Our results encouraged us to disentangle some phylogenetic and taxo- Introduction nomic discrepancies and to improve sebacinalean classifica- tions. This comprises generic circumscriptions and affilia- Based on longitudinally septate meiosporangia in their mature tions, as well as higher taxon groupings. At the family level, stage, sebacinoid fungi were originally grouped together with we redefined the Sebacinaceae, formerly the Sebacinales tremelloid and exidioid taxa. Sebacinales in the present cir- group A, and set it apart from the Sebacinales group B. For cumscription were reviewed in detail recently (Oberwinkler taxonomical purposes, it seems appropriate to refer et al. 2013). We refer to this publication for traditional classi- Paulisebacina, Craterocolla, Chaetospermum, fication of genera and interpretation of some species. Here, we Globulisebacina, Tremelloscypha, and Sebacina to the summarize data that accumulated within several years of Sebacinaceae and Piriformospora, and Serendipita to the intensive sampling, from morphological and molecular stud- Sebacinales group B. -

Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
The Japanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 65 (3): 127–139 (2014) Cephalanthera falcata f. conformis (Orchidaceae) forma nov.: A New Peloric Orchid from Ibaraki Prefecture, Japan 1,* 1 1 HirosHi Hayakawa , CHiHiro Hayakawa , yosHinobu kusumoto , tomoko 1 1 2 3,4 nisHida , Hiroaki ikeda , tatsuya Fukuda and Jun yokoyama 1National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba, Ibaraki 305-8604, Japan. *[email protected] (author for correspondences); 2Faculty of Agriculture, Kochi University, Nan- koku, Kochi 783-8502, Japan; 3Faculty of Science, Yamagata University, Kojirakawa, Yamagata 990-8560, Japan; 4Institute for Regional Innovation, Yamagata University, Kaminoyama, Yamagata 999-3101, Japan We recognize a new peloric form of the orchid Cephalanthera falcata (Thunb.) Blume f. conformis Hi- ros. Hayak. et J. Yokoy., which occurs in the vicinity of the Tsukuba mountain range in Ibaraki Prefec- ture, Japan. This peloric form is sympatric with C. falcata f. falcata. The peloric flowers have a petal-like lip. The flowers are radially symmetrical and the perianth parts spread weakly compared to normal flow- ers. The stigmas are positioned at the column apex, thus neighboring stigmas and the lower parts of the pollinia are conglutinated. We observed similar vegetative traits among C. falcata f. falcata, C. falcata f. albescens S. Kobayashi, and C. falcata f. conformis. The floral morphology in C. falcata f. conformis resembles that of C. nanchuanica (S.C. Chen) X.H. Jin et X.G. Xiang (i.e., similar petal-like lip and stig- ma position at the column apex; syn. Tangtsinia nanchuanica S.C. -

Ectomycorrhizal Fungal Community Structure in a Young Orchard of Grafted and Ungrafted Hybrid Chestnut Saplings
Mycorrhiza (2021) 31:189–201 https://doi.org/10.1007/s00572-020-01015-0 ORIGINAL ARTICLE Ectomycorrhizal fungal community structure in a young orchard of grafted and ungrafted hybrid chestnut saplings Serena Santolamazza‑Carbone1,2 · Laura Iglesias‑Bernabé1 · Esteban Sinde‑Stompel3 · Pedro Pablo Gallego1,2 Received: 29 August 2020 / Accepted: 17 December 2020 / Published online: 27 January 2021 © The Author(s) 2021 Abstract Ectomycorrhizal (ECM) fungal community of the European chestnut has been poorly investigated, and mostly by sporocarp sampling. We proposed the study of the ECM fungal community of 2-year-old chestnut hybrids Castanea × coudercii (Castanea sativa × Castanea crenata) using molecular approaches. By using the chestnut hybrid clones 111 and 125, we assessed the impact of grafting on ECM colonization rate, species diversity, and fungal community composition. The clone type did not have an impact on the studied variables; however, grafting signifcantly infuenced ECM colonization rate in clone 111. Species diversity and richness did not vary between the experimental groups. Grafted and ungrafted plants of clone 111 had a diferent ECM fungal species composition. Sequence data from ITS regions of rDNA revealed the presence of 9 orders, 15 families, 19 genera, and 27 species of ECM fungi, most of them generalist, early-stage species. Thirteen new taxa were described in association with chestnuts. The basidiomycetes Agaricales (13 taxa) and Boletales (11 taxa) represented 36% and 31%, of the total sampled ECM fungal taxa, respectively. Scleroderma citrinum, S. areolatum, and S. polyrhizum (Boletales) were found in 86% of the trees and represented 39% of total ECM root tips. The ascomycete Cenococcum geophilum (Mytilinidiales) was found in 80% of the trees but accounted only for 6% of the colonized root tips. -

Description and Identification of Ostryopsis Davidiana Ectomycorrhizae in Inner Mongolia Mountain Forest of China
Österr. Z. Pilzk. 26 (2017) – Austrian J. Mycol. 26 (2017) 17 Description and identification of Ostryopsis davidiana ectomycorrhizae in Inner Mongolia mountain forest of China QING-ZHI YAO1 WEI YAN2 HUI-YING ZHAO1 JIE WEI2 1 Life Science College 2 Forestry College Inner Mongolia Agriculture University Huhhot, 010018, P. R. China Email: [email protected] Accepted 27. March 2017. © Austrian Mycological Society, published online 23. August 2017 YAO, Q.-Z., YAN, W., ZHAO, H.-Y., WEI, J., 2017: Description and identification of Ostryopsis davidi- ana ectomycorrhizae in Inner Mongolia mountain forest of China. – Austrian J. Mycol. 26: 17–25. Key words: ECM, Mountain forest, Ostryopsis davidiana, morpho-anatomical features. Abstract: The ectomycorrhizal (ECM) fungal composition and anatomical structures of root samples of the shrub Ostryopsis davidiana were examined. The root samples were collected from two plots in the Daqing Mountain and Han Mountain around Hohhot, Inner Mongolia of China. Basing on mor- pho-anatomical features of the samples, we have got totally 12 ECM morphotypes. Twelve fungal taxa were identified via sequencing of the internal transcribed spacer region of their nuclear rDNA. Nine species are Basidiomycotina, incl. Thelephoraceae (Tomentella), Cortinariaceae (Inocybe and Cortinarius), Tremellaceae (Sebacina), Russulaceae (Lactarius), and Tricholomataceae (Tricholoma), three Ascomycotina, incl. Elaphomycetaceae (Cenococcum), Tuberaceae (Tuber), and Pyronema- taceae (Wilcoxina). Cenococcum geophilum was the dominant species in O. davidiana. The three To- mentella and the two Inocybe ECMF of O. davidiana are very common in Inner Mongolia. Zusammenfassung: Die Pilzdiversität der Ektomykorrhiza (ECM) und deren anatomische Strukturen von Wurzelproben des Strauches Ostryopsis davidiana wurden untersucht. Die Wurzelproben wurden aus zwei Untersuchungsflächen im Daqing Berg und Han Berg nahe Hohhot, Innere Mongolei, China, gesammelt. -

Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local Study)
plants Article Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study) Lukáš Wittlinger and Lucia Petrikoviˇcová * Department of Geography and Regional Development, Faculty of Natural Sciences, Constantine the Philosopher University in Nitra, 94974 Nitra, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-907-3441-04 Abstract: In the years 2018–2020, we carried out large-scale mapping in the Western Carpathians with a focus on determining the biodiversity of taxa of the family Orchidaceae using field biogeographical research. We evaluated the research using phytogeographic analysis with an emphasis on selected ecological environmental factors (substrate: ecological land unit value, soil reaction (pH), terrain: slope (◦), flow and hydrogeological productivity (m2.s−1) and average annual amounts of global radiation (kWh.m–2). A total of 19 species were found in the area, of which the majority were Cephalenthera longifolia, Cephalenthera damasonium and Anacamptis morio. Rare findings included Epipactis muelleri, Epipactis leptochila and Limodorum abortivum. We determined the ecological demands of the abiotic environment of individual species by means of a functional analysis of communities. The research confirmed that most of the orchids that were studied occurred in acidified, calcified and basophil locations. From the location of the distribution of individual populations, it is clear that they are generally arranged compactly and occasionally scattered, which results in ecological and environmental diversity. During the research, we identified 129 localities with the occurrence of Citation: Wittlinger, L.; Petrikoviˇcová, L. Phytogeographical Analysis and 19 species and subspecies of orchids. We identify the main factors that threaten them and propose Ecological Factors of the Distribution specific measures to protect vulnerable populations. -

Cotswolds Beechwoods SAC Citation
EC Directive 92/43 on the Conservation of Natural Habitats and of Wild Fauna and Flora Citation for Special Area of Conservation (SAC) Name: Cotswold Beechwoods Unitary Authority/County: Gloucestershire SAC status: Designated on 1 April 2005 Grid reference: SO898134 SAC EU code: UK0013658 Area (ha): 585.85 Component SSSI: Cotswold Commons and Beechwoods SSSI Site description: The site consists of ancient beech woodland and unimproved grassland lying over Jurassic limestones at the western edge of the Cotswolds. The woodlands are amongst the most diverse and species-rich of their type while the grasslands typify the unimproved calcareous pastures for which the area is famous. The woods are structurally varied, including blocks of high forest and some areas of remnant beech coppice. The canopy is dominated by beech Fagus sylvatica, with ash Fraxinus excelsior, pedunculate oak Quercus robur and some areas of sycamore Acer pseudoplatanus. Characteristic understorey species include holly Ilex aquifolium and yew Taxus baccata but regenerating ash, sycamore and beech often accounts for much of the shrub layer. The field layer consists mainly of bramble Rubus fruticosus agg., dog’s mercury Mercurialis perennis and ivy Hedera helix. Rare plants include red helleborine Cephalanthera rubra, stinking hellebore Helleborus foetidus, narrow-lipped helleborine Epipactis leptochila and wood barley Hordelymus europaeus. The fauna of the woods includes an exceptional variety of invertebrate species, including a rich mollusc fauna. The unimproved limestone grassland swards are generally dominated by upright brome Bromopsis erecta, tor-grass Brachypodium pinnatum and sheep’s-fescue Festuca ovina, with quaking grass Briza media and a wide range of other flowering herbs. -

Taxonomy and Systematics of Thelephorales – Glimpses Into Its Hidden Hyperdiversity
Taxonomy and Systematics of Thelephorales – Glimpses Into its Hidden Hyperdiversity Sten Svantesson 2020 UNIVERSITY OF GOTHENBURG Faculty of Science Department of Biological and Environmental Sciences Opponent Prof. Annemieke Verbeken Examiner Prof. Bengt Oxelman Supervisors Associate Prof. Ellen Larsson & Profs. Karl-Henrik Larsson, Urmas Kõljalg Associate Profs. Tom W. May, R. Henrik Nilsson © Sten Svantesson All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without written permission. Svantesson S (2020) Taxonomy and systematics of Thelephorales – glimpses into its hidden hyperdiversity. PhD thesis. Department of Biological and Environmental Sciences, University of Gothenburg, Gothenburg, Sweden. Många är långa och svåra att fånga Cover image: Pseudotomentella alobata, a newly described species in the Pseudotomentella tristis group. Många syns inte men finns ändå Många är gula och fula och gröna ISBN print: 978-91-8009-064-3 Och sköna och röda eller blå ISBN digital: 978-91-8009-065-0 Många är stora som hus eller så NMÄ NENMÄRK ANE RKE VA ET SV T Digital version available at: http://hdl.handle.net/2077/66642 S Men de flesta är små, mycket små, mycket små Trycksak Trycksak 3041 0234 – Olle Adolphson, från visan Okända djur Printed by Stema Specialtryck AB 3041 0234 © Sten Svantesson All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without written permission. Svantesson S (2020) Taxonomy and systematics of Thelephorales – glimpses into its hidden hyperdiversity. PhD thesis. Department of Biological and Environmental Sciences, University of Gothenburg, Gothenburg, Sweden. Många är långa och svåra att fånga Cover image: Pseudotomentella alobata, a newly described species in the Pseudotomentella tristis group.