Modeling of Transient Heat Flux in Spark Ignition Engineduring Combustion and Comparisons with Experiment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

For Sale - Taman Putra Perdana, Puchong, Selangor
iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Sale - Taman Putra Perdana, Puchong, Selangor Reference No: 101636580 Tenure: Leasehold Name: Pun Chee Boon Address: Jalan Putra Perdana 3/11, Occupancy: Tenanted Company: CTG Realty Sdn Bhd Taman Putra Perdana, Taman Furnishing: Unfurnished Email: [email protected] Putra Perdana, 47100, Selangor Unit Type: Intermediate State: Selangor Land Title: Residential Property Type: 2-sty Terrace/Link House Property Title Type: Individual Asking Price: RM 446,666 Posted Date: 13/01/2021 Built-up Size: 1,260 Square Feet Facilities: Parking Built-up Price: RM 354.5 per Square Feet Land Area Size: 18x60 Square Feet No. of Bedrooms: 3+1 No. of Bathrooms: 2 Taman Putra Perdana, 2 Storey End Lot House For Sales; Near Aman Putra and Bandar Nusa Putra; Leaseholde; Land area: 18x60sq.ft.(No Extra Land); Build Up: 1260sq.ft.; 4 rooms + 2 Bathrooms; Basic; Selling Price: RM446,666.66 (nego); Bank Value is RM460k; 10% Deposit & 90% Loan, No Problems. Please kindly call pun 017-6897706 / 016-6005232 for viewing. Taman Putra Perdana is a mature area with all the facilities, it's about 15 years old, neighboring with Cyberjaya and only 6km to Multimedia University. MEX highway are just 1km from Putra Perdana, it's very convenience for those who .... [More] View More Details On iProperty.com iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Sale - Taman Putra Perdana, Puchong, Selangor. -

For Rent - Nilai,Semenyih,Kajang,Puchong,KLIA,Balakong,USJ, Subang Jaya, Nilai, Negeri
iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Rent - Nilai,Semenyih,Kajang,Puchong,KLIA,Balakong,USJ, Subang Jaya, Nilai, Negeri Reference No: 101749154 Tenure: Freehold Address: Nilai, Negeri Sembilan Occupancy: Vacant State: Negeri Sembilan Furnishing: Partly furnished Property Type: Detached factory Unit Type: Intermediate Rental Price: RM 40,000 Land Title: Industrial Built-up Size: 35,000 Square Feet Property Title Type: Individual Built-up Price: RM 1.14 per Square Feet Posted Date: 31/05/2021 Land Area Size: 43,560 Square Feet Land Area Price: RM .92 per Square Feet No. of Bedrooms: 6 Name: Vincent Tan No. of Bathrooms: 6 Company: Gather Properties Sdn. Bhd. Email: [email protected] 2 Storey Detached Factory In Nilai Location: Nilai, Negeri Sembilan - 10 minitues driving distance away from Nilai Toll(Exit PLUS Highway) - 15 minitues driving distance away from Nilai Toll (Exit LEKAS Highway) - 10 minitues driving distance to banks, restaurants, mamak restaurants, malay restaurants, clinics, sundry shops, and others - worker accommodation is easily available. Property details: - 2 Storey office and 1 storey warehouse - Land size: 43,550 sqft - Built up: 36,000 sqft - Freehold - Monthly Rental Price : RM 40,000.00 - The factory is equipped with Certificate Of Fitn.... [More] View More Details On iProperty.com iProperty.com Malaysia Sdn Bhd Level 35, The Gardens South Tower, Mid Valley City, Lingkaran Syed Putra, 59200 Kuala Lumpur Tel: +603 6419 5166 | Fax: +603 6419 5167 For Rent - Nilai,Semenyih,Kajang,Puchong,KLIA,Balakong,USJ, Subang Jaya, Nilai, Negeri. -

Klinik Panel Selangor
SENARAI KLINIK PANEL (OB) PERKESO YANG BERKELAYAKAN* (SELANGOR) BIL NAMA KLINIK ALAMAT KLINIK NO. TELEFON KOD KLINIK NAMA DOKTOR 20, JALAN 21/11B, SEA PARK, 1 KLINIK LOH 03-78767410 K32010A DR. LOH TAK SENG 46300 PETALING JAYA, SELANGOR. 72, JALAN OTHMAN TIMOR, 46000 PETALING JAYA, 2 KLINIK WU & TANGLIM 03-77859295 03-77859295 DR WU CHIN FOONG SELANGOR. DR.LEELA RATOS DAN RAKAN- 86, JALAN OTHMAN, 46000 PETALING JAYA, 3 03-77822061 K32018V DR. ALBERT A/L S.V.NICKAM RAKAN SELANGOR. 80 A, JALAN OTHMAN, 4 P.J. POLYCLINIC 03-77824487 K32019M DR. TAN WEI WEI 46000 PETALING JAYA, SELANGOR. 6, JALAN SS 3/35 UNIVERSITY GARDENS SUBANG, 5 KELINIK NASIONAL 03-78764808 K32031B DR. CHANDRAKANTHAN MURUGASU 47300 SG WAY PETALING JAYA, SELANGOR. 6 KLINIK NG SENDIRIAN 37, JALAN SULAIMAN, 43000 KAJANG, SELANGOR. 03-87363443 K32053A DR. HEW FEE MIEN 7 KLINIK NG SENDIRIAN 14, JALAN BESAR, 43500 SEMENYIH, SELANGOR. 03-87238218 K32054Y DR. ROSALIND NG AI CHOO 5, JALAN 1/8C, 43650 BANDAR BARU BANGI, 8 KLINIK NG SENDIRIAN 03-89250185 K32057K DR. LIM ANN KOON SELANGOR. NO. 5, MAIN ROAD, TAMAN DENGKIL, 9 KLINIK LINGAM 03-87686260 K32069V DR. RAJ KUMAR A/L S.MAHARAJAH 43800 DENGKIL, SELANGOR. NO. 87, JALAN 1/12, 46000 PETALING JAYA, 10 KLINIK MEIN DAN SURGERI 03-77827073 K32078M DR. MANJIT SINGH A/L SEWA SINGH SELANGOR. 2, JALAN 21/2, SEAPARK, 46300 PETALING JAYA, 11 KLINIK MEDIVIRON SDN BHD 03-78768334 K32101P DR. LIM HENG HUAT SELANGOR. NO. 26, JALAN MJ/1 MEDAN MAJU JAYA, BATU 7 1/2 POLIKLINIK LUDHER BHULLAR 12 JALAN KLANG LAMA, 46000 PETALING JAYA, 03-7781969 K32106V DR. -

Residensi Cyberjaya Lakefront
RESIDENSI CYBERJAYA LAKEFRONT perhubungan di antara lebuhraya seperti MEX, ELITE, SKVE & LDP, RESIDENSI memudahkan komuniti mengunjungi pelbagai destinasi dengan pantas terutamanya di sekitar Lembah Klang. Pengangkutan awam yang serba lengkap seperti ERL, dan Cyberjaya DTS Cyberjaya Lakefront menjadikan kediaman ini sebagai destinasi komuniti yang strategik. Apartmen | 1,932 Unit KE PUCHONG/ KE BANDARAYA SUNWAY KE SHAH ALAM/ KUALA LUMPUR KLANG PUCHONG BUKIT JALIL SERDANG KE KAJANG/ SKVE EXPRESSWAY BANGI AY HOTEL UNITEN LIMKOKWING SW UNIVERSITY ES MARRIOTT PR X E X E M SKY SETIA ECO PARK GLADES MEASAT PUTRAJAYA LINGK SENTRAL JALAN PUCHONG AR AN P U T R THE A J PLACE HOSPITAL ELITE HIGHWAY A Y PUTRAJAYA CYBERJAYA A JALAN BARU LAKE GARDEN MULTIMEDIA UNIVERSITY LEBUHRAYA DAMANSARA PUCHONG PERSIARAN SEMARAK API PERSIARAN APEC CENTURY SEPANG SQUARE PERSIARAN PERSIARAN MULTIMEDIA SELANGOR SCIENCE PARK D’PULZE MDEC PUTRAJAYA - CYBERJAYA EXPRESSWAY RESIDENSI CYBERJAYA LAKEFRONT KE KLIA KE DENGKIL/ KE NILAI/ BANTING SEREMBAN 1800-18-1897 Isnin hingga Jumaat (9.00 pagi - 6.00 petang) Sabtu (9.00 pagi - 1.00 petang) E-mel: [email protected] www.pr1ma.my PEMAJU : LAKEFRONT RESIDENCE SDN BHD (934038-V) ALAMAT : GROUND FLOOR, MCT TOWER, ONE CITY, JALAN USJ 25/1, 47650 SUBANG JAYA, SELANGOR Nombor Lesen Pemaju: 12047-2/05-2020/01777 (L) • Tempoh Sahlaku: 23/05/2019-22/05/2020 • Nombor Permit Iklan & Jualan: 12047-2/05-2020/01777 (P) • Tempoh Sahlaku: 23/05/2019-22/05/2020 • Pihak Berkuasa: Majlis Perbandaran Sepang• No. Pelan Bangunan Diluluskan: MPSPG.9/CYB/178/11 -
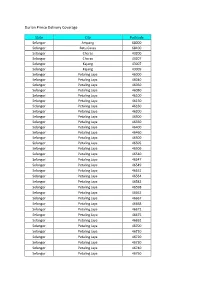
Durian Prince Delivery Coverage
Durian Prince Delivery Coverage State City Postcode Selangor Ampang 68000 Selangor Batu Caves 68100 Selangor Cheras 43200 Selangor Cheras 43207 Selangor Kajang 43007 Selangor Kajang 43009 Selangor Petaling Jaya 46000 Selangor Petaling Jaya 46040 Selangor Petaling Jaya 46050 Selangor Petaling Jaya 46080 Selangor Petaling Jaya 46100 Selangor Petaling Jaya 46150 Selangor Petaling Jaya 46160 Selangor Petaling Jaya 46200 Selangor Petaling Jaya 46300 Selangor Petaling Jaya 46350 Selangor Petaling Jaya 46400 Selangor Petaling Jaya 46460 Selangor Petaling Jaya 46500 Selangor Petaling Jaya 46505 Selangor Petaling Jaya 46506 Selangor Petaling Jaya 46510 Selangor Petaling Jaya 46547 Selangor Petaling Jaya 46549 Selangor Petaling Jaya 46551 Selangor Petaling Jaya 46564 Selangor Petaling Jaya 46582 Selangor Petaling Jaya 46598 Selangor Petaling Jaya 46662 Selangor Petaling Jaya 46667 Selangor Petaling Jaya 46668 Selangor Petaling Jaya 46672 Selangor Petaling Jaya 46675 Selangor Petaling Jaya 46692 Selangor Petaling Jaya 46700 Selangor Petaling Jaya 46710 Selangor Petaling Jaya 46720 Selangor Petaling Jaya 46730 Selangor Petaling Jaya 46740 Selangor Petaling Jaya 46750 Selangor Petaling Jaya 46760 Selangor Petaling Jaya 46770 Selangor Petaling Jaya 46780 Selangor Petaling Jaya 46781 Selangor Petaling Jaya 46782 Selangor Petaling Jaya 46783 Selangor Petaling Jaya 46784 Selangor Petaling Jaya 46785 Selangor Petaling Jaya 46786 Selangor Petaling Jaya 46787 Selangor Petaling Jaya 46788 Selangor Petaling Jaya 46789 Selangor Petaling Jaya 46790 Selangor Petaling -

Invasive Apple Snails in Malaysia
Invasive apple snails in Malaysia H. Yahaya1, A. Badrulhadza2, A. Sivapragasam3, M. Nordin4, M.N. Muhamad Hisham5 and H. Misrudin4 1Paddy and Rice Research Centre, Malaysian Agricultural Research and Development Institute (MARDI), Telong, 16310 Bachok, Kelantan, Malaysia. Email: yahayahussain@ gmail.com, [email protected] (Current address: Lot 241 Kampung Renik Banggu, Jalan Bukit Marak, 16150, Kota Bharu, Kelantan) 2Crop and Soil Science Research Centre, Malaysian Agricultural Research and Development Institute (MARDI), 43400, Serdang, Selangor, Malaysia. Email: bhadza@ mardi.gov.my 3CABI South East Asia, Building A 19, MARDI 43400 Serdang, Selangor, Malaysia. Email: [email protected] 4Crop Protection and Plant Quarantine Division, Department of Agriculture, Kuala Lumpur, Malaysia. Email: [email protected], [email protected] 5Muda Agricultural Development Authority, Alor Setar, Kedah, Malaysia. Email [email protected] Abstract South American apple snails, Pomacea spp., classified as quarantine pests in Malaysia, were first detected in Malaysia in 1991. However, it took almost 10 years before they developed into one of the major pests of rice in the country. Since then, they have spread to almost all the rice areas in Malaysia. Since their detection, continuous control, containment and eradication programmes and research activities have been conducted by various government agricultural agencies involved in rice production. The efforts have been successful in reducing crop damage by the snails but have failed to arrest their dispersal to new areas. Since 2002, the snails have infested almost 20,000 ha of rice growing areas (2008 data) and have threatened the livelihoods of farmers. In 2010, costs associated with apple snail damage were estimated as RM 82 million (US $28 million). -

Construction Aggregate Resources in the Federal Territory and Central Selangor
Geological Society of Malaysia Annual Geological Conference 2000 September 8-9 2000, Pulau Pinang, Malaysia Construction Aggregate Resources in the Federal Territory and Central Selangor CHEONG KHAI WENG & YEAP EE BENG Department of Geology, University of Malaya, 50603 Kuala Lumpur, Malaysia Abstract The Federal Territory of Kuala Lumpur and Selangor have produced 29% of the total crushed rock production in Malaysia. The average consumption per capita in 1998 was 3.74 tonnes of aggregates. It is estimated that the current rock reserve in this area can only cope with the demands of this region for the next 30 years. Thus, the exploitation of aggregate resources must be planned carefully and integrated with other types of landuse. Sumber Agregat Pembinaan di Wilayah Persekutuan dan Selangor Abstrak Wilayah Persekutuan Kuala Lumpur dan Selangor telah menghasilkan 29% daripada jumlah pengeluaran batu hancur di Malaysia. Jumlah penggunaan agregat per kapita pada tahun 1998 adalah 3.74 ton. Dianggarkan simpanan batuan sedia ada di kawasan ini hanya boleh memenuhi keperluan rantau ini untuk 30 tahun akan datang. Maka, eksploitasi sumber agregat mestilah dirancang dengan teliti dan disepadukan dengan jenis gunatanah yang lain. INTRODUCTION and provide plentiful construction aggregates to the Hulu Langat - Semenyih area. The areas around the Lagong Coarse aggregate is one of the most accessible natural Forest Reserve in the District of Gombak has currently industrial material and a major basic raw material used attracted a lot of quarry operators. One granite quarry in by the construction industry. It consists of crushed stone, Bukit Lanchong is stragetically located in a highly which is defined as "the product resulting from artificial populated area. -

6 Fire Rescue Divers Killed in the Line of Duty During Water Rescue
10-03-2018 – Malaysia - 6 FIRE RESCUE DIVERS KILLED – FF PSD 6 Fire Rescue Divers Killed In The Line Of Duty During Water Rescue https://www.firefighterclosecalls.com/6-fire-rescue-divers-killed-in-the-line-of-duty-during-water-rescue/ October 4, 2018 We regret to pass on that 6 fire and rescue department divers in Malaysia died in the Line of Duty. This occurred during a rescue operation for a teenager who is feared to have drowned after falling into a mining pond on Wednesday (Oct 3). The divers were caught in a strong “whirlpool” during the operation in Puchong, a town on the outskirts of Kuala Lumpur. It was drizzling when the divers went into the pond at 2115 hours to rescue the 17- year-old boy. The team had followed standard operating procedures in donning complete diving equipment and were tied to a single rope. PSDiver Magazine www.PSDiver.com Page 1 10-03-2018 – Malaysia - 6 FIRE RESCUE DIVERS KILLED – FF PSD Suddenly a violent current/whirlpool occurred in the water, causing all the fire rescue divers to spin in the water while all their equipment came off. The divers struggled in the water for about 30 minutes while other fire rescue members tried to rescue them. All of them were unconscious when they were eventually pulled out of the water. All life saving measures were attempted, but unsuccessful. Here’s What Happened to 6 Firemen Who Drowned While Finding Missing Teen in Puchong https://www.worldofbuzz.com/heres-what-happened-to-6-firemen-who-drowned-while-finding- missing-teen-in-puchong/ October 4, 2018 By Ling Kwan On the night of 3 October, the nation was shocked by news of six firemen who drowned while carrying out a search and rescue operation. -

Subang Jaya, Malaysia – an Action Plan Towards Adequate Housing for All”
Affordable Living in Sustainable Cities Congress Newcastle NSW 2018 “TOD Initiatives in the City of Subang Jaya, Malaysia – An Action Plan towards Adequate Housing for All” By KHAIRIAH TALHA Hon. President EAROPH 1 SUBANG JAYA CITY PROFILE 2 KEY PLAN LOCATION PLAN PERAK Perlis THAILAND Kedah Sabak PAHANG Bernam Hulu P.Pinang Selangor Kelantan Kuala Terengganu Laut China Selangor Perak Selatan Gombak KUALA Pahang PetalingLUMPUR Hulu Langat Selangor Klang MPSJ MPSJ K.Lumpur Putrajaya N.Sembilan Kuala Langat NEGERI Melaka Sepang SEMBILAN Johor INDONESIA SINGAPURA 3 ADJOINING DEVELOPMENTS 4 POPULATION MPSJ TOTAL AREA PROJECTION 2015 - 2035 CURRENT YEAR 2015 POPULATION 798,830 YEAR 2020 968,930 16,180.00 YEAR 2025 HECTARE 1,161,513 YEAR 2030 1,349,841 YEAR 2035 1,556,6565 POPULATION PROJECTION FOR MPSJ 2015 - 2035 900,000 800,000 700,000 600,000 500,000 400,000 300,000 200,000 100,000 0 YEAR 2010 YEAR 2015 YEAR 2020 YEAR 2025 YEAR 2030 YEAR 2035 MALE FEMALE GENDER 2010 % 2015 % 2020 % 2025 % 2030 % 2035 % MALE 335,567 51.24 406,636 50.90 490,031 50.57 583,920 50.27 673,885 49.92 772,057 49.60 FEMALE 319,385 48.76 392,194 49.10 478,899 49.43 577,592 49.73 675,957 50.08 784,599 50.40 Total 654,952 100.00 798,829 100.00 968,930 100.00 1,161,512 100.00 1,349,842 100.00 1,556,656 100.006 CURRENT LANDUSE ( 2015) EXISTING HOUSING ( 2015) HIGH-RISE HOUSING, 9.33 HOUSING / RESIDENTIAL LOW COST TOWN HOUSE, 25% CLUSTER, 0.07 HIGH-RISE, 3.68 TRANSPORTATION 0.47 26% TERREACE HOUSING, GOVERNMENT 40.77 QUARTER, 0.03 OTHERS, 11.78 WATER BODIES 1% VACANT LAND -

1970 Population Census of Peninsular Malaysia .02 Sample
1970 POPULATION CENSUS OF PENINSULAR MALAYSIA .02 SAMPLE - MASTER FILE DATA DOCUMENTATION AND CODEBOOK 1970 POPULATION CENSUS OF PENINSULAR MALAYSIA .02 SAMPLE - MASTER FILE CONTENTS Page TECHNICAL INFORMATION ON THE DATA TAPE 1 DESCRIPTION OF THE DATA FILE 2 INDEX OF VARIABLES FOR RECORD TYPE 1: HOUSEHOLD RECORD 4 INDEX OF VARIABLES FOR RECORD TYPE 2: PERSON RECORD (AGE BELOW 10) 5 INDEX OF VARIABLES FOR RECORD TYPE 3: PERSON RECORD (AGE 10 AND ABOVE) 6 CODES AND DESCRIPTIONS OF VARIABLES FOR RECORD TYPE 1 7 CODES AND DESCRIPTIONS OF VARIABLES FOR RECORD TYPE 2 15 CODES AND DESCRIPTIONS OF VARIABLES FOR RECORD TYPE 3 24 APPENDICES: A.1: Household Form for Peninsular Malaysia, Census of Malaysia, 1970 (Form 4) 33 A.2: Individual Form for Peninsular Malaysia, Census of Malaysia, 1970 (Form 5) 34 B.1: List of State and District Codes 35 B.2: List of Codes of Local Authority (Cities and Towns) Codes within States and Districts for States 38 B.3: "Cartographic Frames for Peninsular Malaysia District Statistics, 1947-1982" by P.P. Courtenay and Kate K.Y. Van (Maps of Adminsitrative district boundaries for all postwar censuses). 70 C: Place of Previous Residence Codes 94 D: 1970 Population Census Occupational Classification 97 E: 1970 Population Census Industrial Classification 104 F: Chinese Age Conversion Table 110 G: Educational Equivalents 111 H: R. Chander, D.A. Fernadez and D. Johnson. 1976. "Malaysia: The 1970 Population and Housing Census." Pp. 117-131 in Lee-Jay Cho (ed.) Introduction to Censuses of Asia and the Pacific, 1970-1974. Honolulu, Hawaii: East-West Population Institute. -

Putraresidence E-Brochure.Pdf
Developing Homes, Building Lifestyles At Sime Darby Property, we do not merely build houses, we design homes that complement the way you live life. From the distinct townships where these homes are built to the exclusive features that come with each property, our homes are an extension of your personality and lifestyle. Ranging from bungalows with large open spaces to cosy serviced apartments perfect for two, you will find your ideal Sime Darby Property home that reflects who you are and what you aspire to in life. A Lakeside Enclave Adjacent to LRT stations Where Serenity & Accessibility Intertwine Artist's Impression LRT From Shah Alam / KL Station Kelana Jaya Line am g From Shah Al (NKVE From Suban Sheridan ) L D P ( L e b u h Estelina r USJ a UEP y Toll a Subang Jaya Plaza D a m a n s a r To P a ersiaran P Putra Heights u Adelina ch on LRT Pu g) The Heart of Majestic Living tr Station a Bistari Calista Sek. Ren. From Puchong SRLJ (C) USJ Interchange Imagine yourself at the centre of everything that matters. On the pulse of the best things life has USJ 23 Jasmine to offer. In a serene paradise that’s so near, yet so far from the madding crowd. Saya Elite Hightway A strategic location served by the Putra Heights Interchange, USJ Interchange, NKVE, LDP, ELITE, KESAS, SKVE and Federal Highways, where accessibility is king. A beautiful locale Mascarena Palm Carmen USJ 26 Grandis surrounded by the established neighbourhoods of Puchong, Shah Alam, Subang, Klang, Palm Sg. -

Corporate Brochure Eng 2017
DRIVEN BY INNOVATION Engineering Construction Property Development Infrastructure GAMUDA BERHAD (29579-T) Menara Gamuda, Block D, PJ Trade Centre Concessions No. 8, Jalan PJU 8/8A, Bandar Damansara Perdana 47820 Petaling Jaya, Selangor Darul Ehsan, Malaysia 603-7491 8288 603-7728 6571 / 9811 gamuda.com.my Copyright © 2017 by Gamuda Berhad All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form without the prior written permission printed 2017 Starting o as a small construction outt in 1976, we have steadily built our expertise in three core businesses; Engineering & Construction, Property Development and Infrastructure Concessions, achieving a market capitalisation of RM13 billion in 2017. Today, we are proud to be identied as an innovative builder behind some of the world’s rsts, namely the dual purpose Bridges Stormwater Management and Road Tunnel (SMART) and Railway Malaysia’s rst MRT. Systems and Trains IBS Highways and Airport Ports Expressways Hospital Water Tunnelling Treatment Plants 1 Dams Townships Buildings Power Plants 2 9.7km dual-purpose EMBRACING INNOVATION SMART, the world’s rst EXPERT TUNNELLERS With proven expertise and extensive Stormwater management know-how in advanced tunnelling technology and techniques, we have and motorway demonstrated that tunnels need not be single-function structures. This is evident in our track record of constructing tunnels for river and stormwater diversion as well as highways and railways in dicult geological conditions. Listed by CNN as one of the world’s Top 10 greatest tunnels 3 4 Malaysia’s only TBM Refurbishment Plant Using the Tunnel Boring Machine (TBM) under Kuala Lumpur city centre for the SMART project has led to another innovative invention Highly-complex that was successfully used in TBMs used for Malaysia’s rst MRT.