PHEROMONE-BASED MONITORING of Pseudococcus Maritimus and INTRA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abiotic and Biotic Pest Refuges Hamper Biological Control of Mealybugs in California Vineyards K.M
____________________________________ Abiotic and biotic pest refuges in California vineyards 389 ABIOTIC AND BIOTIC PEST REFUGES HAMPER BIOLOGICAL CONTROL OF MEALYBUGS IN CALIFORNIA VINEYARDS K.M. Daane,1 R. Malakar-Kuenen,1 M. Guillén,2 W.J. Bentley3, M. Bianchi,4 and D. González,2 1 Division of Insect Biology, University of California, Berkeley, California, U.S.A. 2 Department of Entomology, University of California, Riverside, California, U.S.A. 3 University of California Statewide IPM Program, Kearney Agricultural Center, Parlier, California, U.S.A. 4 University of California Cooperative Extension, San Luis Obispo, California, U.S.A. INTRODUCTION Four mealybug species cause economic damage in California vineyards. These are the grape mealy- bug, Pseudococcus maritimus (Ehrhorn); obscure mealybug, Pseudococcus viburni (Signoret); longtailed mealybug, Pseudococcus longispinus (Targioni-Tozzeti); and vine mealybug, Planococcus ficus (Signoret) (Godfrey et al., 2002). The grape, obscure, and longtailed mealybugs belong to the Pseudococcus maritimus-malacearum complex–a taxonomically close group of mealybugs (Wilkey and McKenzie, 1961). However, while the origins of the grape and longtailed mealybugs are believed to be in North America, the ancestral lines of the obscure mealybug are unclear. Regardless, these three species have been known as pests in North America for nearly 100 years. The vine mealybug, in contrast, was first identified in California in the Coachella Valley in the early 1990s (Gill, 1994). It has since spread into California’s San Joaquin Valley and central coast regions, with new infestations reported each year. The four species are similar in appearance; however, mealybugs in the P. maritimus- malacearum complex have longer caudal filaments than vine mealybug (Godfrey et al., 2002). -

Review of Ecologically-Based Pest Management in California Vineyards
insects Review Review of Ecologically-Based Pest Management in California Vineyards Houston Wilson 1,* and Kent M. Daane 2 ID 1 Department of Entomology, University of California, Riverside, Riverside, CA 92521, USA 2 Department Environmental Science, Policy and Management, University of California, Berkeley, Berkeley, CA 94720-3114, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-559-646-6519 Academic Editors: Alberto Pozzebon, Carlo Duso, Gregory M. Loeb and Geoff M. Gurr Received: 28 July 2017; Accepted: 6 October 2017; Published: 11 October 2017 Abstract: Grape growers in California utilize a variety of biological, cultural, and chemical approaches for the management of insect and mite pests in vineyards. This combination of strategies falls within the integrated pest management (IPM) framework, which is considered to be the dominant pest management paradigm in vineyards. While the adoption of IPM has led to notable and significant reductions in the environmental impacts of grape production, some growers are becoming interested in the use of an explicitly non-pesticide approach to pest management that is broadly referred to as ecologically-based pest management (EBPM). Essentially a subset of IPM strategies, EBPM places strong emphasis on practices such as habitat management, natural enemy augmentation and conservation, and animal integration. Here, we summarize the range and known efficacy of EBPM practices utilized in California vineyards, followed by a discussion of research needs and future policy directions. EBPM should in no way be seen in opposition, or as an alternative to the IPM framework. Rather, the further development of more reliable EBPM practices could contribute to the robustness of IPM strategies available to grape growers. -

Evaluation of RNA Interference for Control of the Grape Mealybug Pseudococcus Maritimus (Hemiptera: Pseudococcidae)
insects Article Evaluation of RNA Interference for Control of the Grape Mealybug Pseudococcus maritimus (Hemiptera: Pseudococcidae) Arinder K. Arora 1, Noah Clark 1, Karen S. Wentworth 2, Stephen Hesler 2, Marc Fuchs 3 , Greg Loeb 2 and Angela E. Douglas 1,4,* 1 Department of Entomology, Cornell University, Ithaca, NY 14850, USA; [email protected] (A.K.A.); [email protected] (N.C.) 2 Department of Entomology, Cornell University, Geneva, NY 14456, USA; [email protected] (K.S.W.); [email protected] (S.H.); [email protected] (G.L.) 3 School of Integrative Plant Science, Cornell University, Geneva, NY 14456, USA; [email protected] 4 Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA * Correspondence: [email protected] Received: 19 September 2020; Accepted: 26 October 2020; Published: 28 October 2020 Simple Summary: RNA interference (RNAi) is a defense mechanism that protects insects from viruses by targeting and degrading RNA. This feature has been exploited to reduce the expression of endogenous RNA for determining functions of various genes and for killing insect pests by targeting genes that are vital for insect survival. When dsRNA matching perfectly to the target RNA is administered, the RNAi machinery dices the dsRNA into ~21 bp fragments (known as siRNAs) and one strand of siRNA is employed by the RNAi machinery to target and degrade the target RNA. In this study we used a cocktail of dsRNAs targeting grape mealybug’s aquaporin and sucrase genes to kill the insect. Aquaporins and sucrases are important genes enabling these insects to maintain water relations indispensable for survival and digest complex sugars in the diet of plant sap-feeding insects, including mealybugs. -

An Investigation Into the Integrated Pest Management of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

Transmission of Grapevine Leafroll-Associated Virus 3 (Glrav-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus Ficus and Pseudococcus Longispinus (Hemiptera
Transmission of Grapevine Leafroll-associated Virus 3 (GLRaV-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae) K. Krüger1,*, D.L. Saccaggi1,3, M. van der Merwe2, G.G.F. Kasdorf2 (1) Department of Zoology & Entomology, University of Pretoria, Private Bag X20, Pretoria, 0028, South Africa (2) ARC-Plant Protection Research Institute, Private Bag X134, Pretoria, 0001, South Africa (3) Current address: Plant Health Diagnostic Services, Department of Agriculture, Forestry and Fisheries, Private Bag X5015, Stellenbosch, 7599, South Africa Submitted for publication: December 2014 Accepted for publication: March 2015 Key words: Ampelovirus, Closteroviridae, Coccoidea, grapevine leafroll disease, Vitis vinifera The vine mealybug, Planococcus ficus (Signoret), and the longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti), are vectors of grapevine leafroll-associated virus 3 (GRLaV-3), one of the most abundant viruses associated with grapevine leafroll disease. To elucidate the transmission biology in South Africa, acquisition access periods (AAPs), inoculation access periods (IAPs) and the retention of the virus in starving and feeding first- to second instar nymphs were determined. The rootstock hybrid LN33 served as virus source and grapevines (Vitis vinifera L., cv. Cabernet franc) served as recipient plants. An AAP of 15 min or an IAP of 15 min was sufficient forPl. ficus to acquire or transmit GLRaV-3, respectively. Nymphs of Pl. ficus retained the virus for at least eight days when feeding on a non-virus host and grapevine, and for at least two days when starving, and were then capable of transmitting it successfully to healthy grapevine plants. Nymphs of Ps. longispinus transmitted the virus after an AAP of 30 min and an IAP of 1 h. -

Little Cherry Virus 1 & 2
WASHINGTON STATE UNIVERSITY EXTENSION Little Cherry Virus 1 & 2 Written by: Scott Harper, WSU Plant Pathology; Andrea Bixby Brosi, Betsy Beers, WSU Entomology; Tianna DuPont, WSU Extension. May 2019. Little Cherry Disease (LCD) is a critical concern to sweet cherry producers in the state of Washington. This disease is caused by infection by Little cherry virus 1 (LChV1), Little cherry virus 2 (LChV2) or the X-Disease Phytoplasma (Candidatus Phytoplasma pruni), and produces small, undersized cherries, with poor color development and flavor. Background Little Cherry Disease is not a new problem in the Pacific Figure 2 Little Cherry Virus 2 produces cherries of small size and poor Northwest. The first major epidemic occurred in the Kootenay color and flavor. Photo credit Andrea Bixby Brosi, WSU. valley in Canada in 1938, and over the following 40 years Little cherry virus (both -1 and-2) infection of sweet cherry devastated the Canadian cherry industry so much that by 1979 cultivars results in small fruit with reduced sugar content that the last packing line in the Kootenay valley closed, and the may taste bland or insipid. Severity of the disease differs Canadian industry has spent the last 40 years rebuilding. between cultivars, with Lambert and Bing being highly In Washington State the last major epidemic occurred in the late susceptible, whereas Van and Sam are less so and fruit may 1940s and into the 1950s, resulting in the removal of significant reach marketable size although flavor is still affected. On some acreage. But, with the passage of time and changes in cultivars such as Lambert, the fruit may also have a noticeable management practices, the disease has become prevalent in pointed tip. -
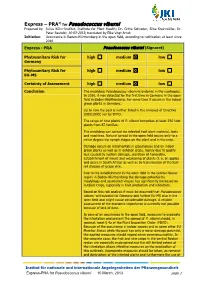
Express PRA Pseudococcus Viburni
1) Express – PRA for Pseudococcus viburni Prepared by: Julius Kühn-Institut, Institute for Plant Health; Dr. Gritta Schrader, Silke Steinmöller, Dr. Peter Baufeld; 10-03-2013; translated by Elke Vogt-Arndt Initiation: Occurrence in Baden-Württemberg in the open field, according to notification at least since 2010 Express - PRA Pseudococcus viburni (Signoret) Phytosanitary Risk for high medium low Germany Phytosanitary Risk for high medium low EU-MS Certainty of Assessment high medium low Conclusion The mealybug Pseudococcus viburni is endemic in the neotropics. In 2010, it was detected for the first time in Germany in the open field in Baden-Württemberg. For some time it occurs in the indoor green plants in Germany. Up to now the pest is neither listed in the annexes of Directive 2000/29/EC nor by EPPO. The range of host plants of P. viburni comprises at least 296 host plants from 87 families. The mealybug can spread via infested host plant material, tools and machines. Natural spread in the open field occurs only to a minor degree via nymph stages on the plant and in the crop. Damage occurs on ornamentals in glasshouses and on indoor green plants as well as in outdoor crops, mainly due to quality loss caused by suction damage, secretion of honeydew, establishment of mould and weakening of plants (f. e. on apples and pears in South Africa) as well as by transmission of the leaf- roll disease of grape vine. Due to the establishment in the open field in the central Neckar region in Baden-Württemberg the damage potential by mealybugs and associated viruses has significantly increased on outdoor crops, especially in fruit production and viticulture. -

Hemiptera: Pseudococcidae) Diversity in Received: 5 April 2017 Accepted: 1 November 2017 Southern Brazilian Fruit Crops Published: Xx Xx Xxxx Vitor C
www.nature.com/scientificreports OPEN Integrative taxonomy methods reveal high mealybug (Hemiptera: Pseudococcidae) diversity in Received: 5 April 2017 Accepted: 1 November 2017 southern Brazilian fruit crops Published: xx xx xxxx Vitor C. Pacheco da Silva1, Mehmet Bora Kaydan2,3, Thibaut Malausa4, Jean-François Germain 5, Ferran Palero4,6 & Marcos Botton7 The Serra Gaúcha region is the most important temperate fruit-producing area in southern Brazil. Despite mealybugs (Hemiptera: Pseudococcidae) infesting several host plants in the region, there is a lack of information about the composition of species damaging diferent crops. A survey of mealybug species associated with commercial fruit crops (apple, persimmon, strawberry and grapes) was performed in Serra Gaúcha between 2013 and 2015, using both morphology and DNA analyses for species identifcation. The most abundant species were Pseudococcus viburni (Signoret), found on all four host plant species, and Dysmicoccus brevipes (Cockerell), infesting persimmon, vines and weeds. The highest diversity of mealybug species was found on persimmon trees, hosting 20 diferent taxa, of which Anisococcus granarae Pacheco da Silva & Kaydan, D. brevipes, Pseudococcus sociabilis Hambleton and Ps. viburni were the most abundant. A total of nine species were recorded in vineyards. Planococcus fcus (Signoret) and Pseudococcus longispinus (Targioni Tozzetti) were observed causing damage to grapes for the frst time. A single species, Ps. viburni, was found associated with apples, while both Ps. viburni and Ferrisia meridionalis Williams were found on strawberry. Four of the mealybug species found represent new records for Brazil. Brazil is the third largest fruit producer in the world, with a cultivated area of 2.5 million hectares and an esti- mated production of 40 million tons1,2. -

SCI Insectsurveys Report.Fm
Terrestrial Invertebrate Survey Report for San Clemente Island, California Final June 2011 Prepared for: Naval Base Coronado 3 Wright Avenue, Bldg. 3 San Diego, California 92135 Point of Contact: Ms. Melissa Booker, Wildlife Biologist Under Contract with: Naval Facilities Engineering Command, Southwest Coastal IPT 2739 McKean Street, Bldg. 291 San Diego, California 92101 Point of Contact: Ms. Michelle Cox, Natural Resource Specialist Under Contract No. N62473-06-D-2402/D.O. 0026 Prepared by: Tierra Data, Inc. 10110 W. Lilac Road Escondido, CA 92026 Points of Contact: Elizabeth M. Kellogg, President; Scott Snover, Biologist; James Lockman, Biologist COVER PHOTO: Halictid bee (Family Halictidae), photo by S. Snover. Naval Auxiliary Landing Field San Clemente Island June 2011 Final Table of Contents 1.0 Introduction . .1 1.1 Regional Setting ................................................................................................................... 1 1.2 Project Background .............................................................................................................. 1 1.2.1 Entomology of the Channel Islands .................................................................... 3 1.2.2 Feeding Behavior of Key Vertebrate Predators on San Clemente Island ............. 4 1.2.3 Climate ................................................................................................................. 4 1.2.4 Island Vegetation.................................................................................................. 5 -

Scale Insect Pests of Connecticut Trees and Ornamentals
Dr. Hugh Smith, Dr. Richard Cowles, and Rose Hiskes Department of Entomology The Connecticut Agricultural Experiment Station 123 Huntington Street, P. O. Box 1106 New Haven, CT 06504 Phone: (203) 974-8600 Fax: (203) 974-8502 Founded in 1875 Email: [email protected] Putting science to work for society Website: www.ct.gov/caes Scale Insect Pests of Connecticut Trees and Ornamentals Scale insects are among the most difficult mealybug pests of Connecticut ornamentals pests of woody ornamentals to manage. include the taxus mealybug on yew, and the There are several types of scale insect, apple mealybug and the Comstock although the most common scale pests for mealybug, both of which attack many hosts. Connecticut growers are armored scales Common scale pests of Connecticut and (Diaspididae), soft scales (Coccidae) and their hosts are presented in Table 1. mealybugs (Pseudococcidae). Armored scales tend to be smaller (2-3 mm) than soft Life cycle. Depending on the species, scale scales (5-10 mm) and often appear as though insects can overwinter as eggs, immatures, they are part of the plant, making them or adults. Females lay eggs beneath the difficult to detect. Armored scales secrete a scale covering or in a cottony mass. Some hard covering that helps protect them from scale species have one generation per year; insecticides and natural enemies, and some species have a few or several prevents them from drying out. This generations per year. First stage immatures, covering can be pried away from the scale called crawlers, emerge from eggs in spring body with the tip of a blade or an insect pin. -

The Structure and Development of Wax Glands of Pseudococcus Maritimus (Homoptera, Coccidae)
The Structure and Development of Wax Glands of Pseudococcus maritimus (Homoptera, Coccidae). By Priscilla Frew Pollister, Department of Zoology, Columbia University. With Plates 17-20, and 3 Text-figures. INTRODUCTION. ONE of the striking features of the family Coccidae is a uni- versal tendency to form extensive secretions of wax or wax-like substances on the surface of the body. This substance seems to serve a protective role, but in many instances the amount of it is so out of proportion to the necessity for this purpose that many entomologists have held that the exudation of this wax is more accurately to be regarded as a type of excretion of a material that is an inevitable by-product of the metabolism of animals feeding exclusively on plant juices. The wax is secreted through pores in the cuticula, which are the openings from underlying glands. The present study is concerned with the distribution, minute structure, and develop- ment of these glands in one of the mealy-bugs, Pseudococcus maritimus Erhorn. There have been many descriptions of the structure and distribution of the external pores, and a few studies of the histology and development of the underlying glands. Many of these observations have been incidental to a work primarily concerned with the taxonomy of the group. The present study attempts to deal with the problem primarily from a histological and cytological point of view, since in these structures one finds some of the most elaborately differentiated types of glands. The problem was suggested by Professor Franz Schrader, and I am indebted to him for advice and encouragement throughout the work. -

First Record of Pseudococcus Jackbeardsleyi (Hemiptera: Pseudococcidae) from Africa, Côte D’Ivoire
1690 Florida Entomologist 97(4) December 2014 FIRST RECORD OF PSEUDOCOCCUS JACKBEARDSLEYI (HEMIPTERA: PSEUDOCOCCIDAE) FROM AFRICA, CÔTE D’IVOIRE 1* 2 3 4 PIERRE WALET N’GUESSAN , GILLIAN W. WATSON , JUDITH K. BROWN AND FRANÇOIS K. N’GUESSAN 1,4Programme cacao, Centre National de Recherche Agronomique, BP 808 Divo, Côte d’Ivoire 2Plant Pest Diagnostic Center, California Department of Food & Agriculture, Sacramento, California, USA 3School of Plant Sciences, The University of Arizona, Tucson, Arizona, USA *Corresponding author; E-mail: [email protected] ABSTRACT Cocoa swollen shoot virus disease is an important virus disease of cocoa occurring mainly in West Africa. Mealybugs are known to be vectors of the pathogen, Cacao swollen shoot virus. Since recent outbreaks in Côte d’Ivoire, studies have been undertaken on different aspects of the disease. During 2013, surveys were conducted to identify mealybug species infesting aer- ial parts of cocoa (Theobroma cacao L.; Malvales: Malvaceae) trees at various sites and the samples were authoritatively identified using morphological characters. A species new to Af- rica, Pseudococcus jackbeardsleyi Gimpel & Miller (Hemiptera: Pseudococcidae), was found at 2 localities in Côte d’Ivoire, i.e., Buyo (Soubré county) and Gbalékro (Agboville county). Hosts of this polyphagous mealybug belong to 47 plant families and include banana, egg- plant, Hibiscus spp., potato, sweet pepper and tomato. Virus transmission by P. jackbeard- sleyi has not been recorded, but it belongs to the same genus as P. maritimus (Ehrhorn), which transmits Little cherry virus 2 to sweet cherry, and P. longispinus (Targioni Tozzetti), which transmits Grapevine A trichovirus (GAV) to grapevine and Cacao swollen shoot virus (CSSV) to cocoa.